Abstract
Tumor necrosis factor receptor‐associated factor‐6 (TRAF6) is a ubiquitin E3 ligase. TRAF6 plays an important role in tumor invasion and metastasis. However, the specific mechanism by which TRAF6 promotes colorectal cancer (CRC) metastasis is incompletely understood. This study aimed to determine whether TRAF6 affects the LPS‐NF‐κB‐VEGF‐C signaling pathway through ubiquitination, which plays a role in colorectal cancer metastasis. Here, our results showed that TRAF6 affected lymphangiogenesis through the LPS‐NF‐κB‐VEGF‐C signaling pathway. Using ubiquitination experiments, we found that TRAF6 was mainly ubiquitinated with the K63‐linked chains, and LPS promoted ubiquitination of TRAF6 and K63‐linked chains. More importantly, TRAF6 124mut is the main ubiquitination site of TRAF6 interacting with K63‐linked chains. TRAF6 affected the migration, invasion, and lymphatic metastasis of colorectal cancer through its ubiquitination. In subcutaneous xenograft models, TRAF6 124mut inhibited tumor growth. In conclusion, our results provide new insight for studying the mechanism of lymphangiogenesis in colorectal cancer to promote cancer metastasis, which may provide new ideas for tumor immunotherapy.
Keywords: colorectal cancer, lymphangiogenesis, NF‐κB, TRAF6, ubiquitination
TRAF6 regulates the LPS‐NF‐kappaB‐VEGF‐C signal pathway to effect colorectal cancer lymphangiogenesis, growth and invasion via a ubiquitination mechanism. Our results provide new insights into the mechanism of colorectal cancer metastasis, which may provide novel ideas for tumor immunotherapy.

1. INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the world and the second leading cause of cancer‐related deaths worldwide. 1 Surgical resection is still the most important treatment method for improving the 5‐year survival rate of patients with CRC. However, 50% of patients with CRC eventually experience relapse and metastasis, shortening the patients’ survival and affecting their prognosis. 2 , 3 Lymphatic metastasis is the main route of CRC metastasis, which is the most important cause of death in patients with tumor disease. 4 , 5 Neolymphangiogenesis of CRC is essential for lymphatic metastasis of CRC. 6 , 7 The mechanism of lymphangiogenesis is incompletely understood. Therefore, exploring the molecular mechanism of lymphatic metastasis of colorectal cancer is important for further understanding this disease and to design of more effective therapies.
Our previous study showed that lipopolysaccharide (LPS) was significantly increased in colorectal cancer patients. The concentration of LPS was higher in colorectal cancer tissues with lymphatic metastasis than without lymphatic metastasis, and we previously found that LPS promotes colorectal cancer micro‐lymph by upregulating the expression of vascular endothelial growth factor‐C (VEGF‐C). It also promotes migration and invasion of colorectal cancer cells. 8 , 9 LPS promoted the secretion of VEGF‐C by colorectal cancer cells via the toll‐like receptor 4 (TLR4)‐NF‐kappaB signaling pathway. 9 However, it has been found that TRAF6 plays an important physiological role in the transduction of LPS‐mediated TLR2 and TLR4 downstream signaling. 10 In colorectal cancer, whether LPS participates in the NF‐κB‐VEGF‐C signaling pathway through other signaling molecules remains to be further studied.
Tumor necrosis factor receptor‐associated factor‐6 (TRAF6) is rapidly recruited and activated by binding of LPS to TLR4. TRAF6 regulates the LPS‐TLR4 signaling pathway both upstream and downstream. 11 Zhou et al. reported that TRAF6 caused changes in the release of pro‐inflammatory cytokines through ubiquitination and activation of downstream pathways. 12 Moreover, PHLDA1 mediated the ubiquitination of TRAF6 and K63 to activate the NF‐κB signaling pathway by influencing LPS. 13 Is TRAF6 involved in the LPS‐NF‐κB‐VEGF‐C signaling pathway related to its ubiquitination?
In the present study, we demonstrated that TRAF6 mediating the LPS‐NF‐κB‐VEGF‐C signaling pathway could enhance colorectal cancer lymphangiogenesis. We also showed that TRAF6 regulated tumor growth and invasion via the ubiquitination mechanism.
2. MATERIAL AND METHODS
2.1. Clinical samples and paraffin tissue section
We collected 100 human colorectal tumor tissue and adjacent corresponding non‐tumor tissue samples from patients who had colorectal surgery at the First Affiliated Hospital of Fujian Medical University (Fuzhou, China) in 2010–2012. The samples were collected from 51 men and 49 women. Among them, 56 cases had lymph node metastasis, 41 cases had 1–3 lymph node metastasis, and 15 cases had ≥4 lymph node metastasis. All patients with colorectal cancer were diagnosed by pathologists. Tissue collection was performed with the patient’s informed consent. The study was approved by the Institutional Review Board (IRB) of the Hospital. Patients with microsatellite instability and familial and genetic predisposition to CRC were excluded. The patients did not receive chemotherapy or radiotherapy before surgery.
2.2. Tissue immunohistochemistry and evaluation
The UltraSensitiveTM SP (Mouse/Rabbit) IHC Kit (MXB biotechnologies) was used, and immunohistochemistry was performed according to the manufacturer’s instructions. Serial tissue sections were obtained from formalin‐fixing and paraffin‐embedding specimens. The sections were incubated in a baking machine (LEICA HI1220, Laica, Germany) for 30 min at 65℃. Tissue antigen was protected with an endogenous peroxidase blocking solution. After washing with tris‐buffered saline Tween (TBST) for 5 min, the slices were blocked with skimmed milk power (BD, USA) for 10 min at 37℃. Two serial sections were incubated in a 1:500 dilution of rabbit polyclonal anti‐human/anti‐mouse TRAF6 antibody (Affinity), a 1:100 dilution of mouse monoclonal anti‐human D2‐40 antibody (Sigma‐Aldrich), or a 1:100 dilution of rabbit polyclonal anti‐mouse LYVE‐1 antibody (Affinity) overnight at 4℃. For negative control, TBST was substituted for the primary antibody. A bio‐conjugated second antibody and streptavidin‐peroxidase were then added. A DAB solution was used for color development. After hematoxylin staining, the slides were observed with a microscope. Image‐Pro Plus 6.0 software (Media Cybernatics) was used to evaluate the expression of TRAF6. Three sections were randomly selected from each group, and five fields were randomly selected from each section. The expression of TRAF6 was quantified by mean optical density (MOD) = the integral optical density/area. Lymphatic vessel density (LVD) was determined by counting the number of D2‐40 or LYVE‐1‐positive vessels per HPF (100×) in the tumor.
2.3. Cell culture
SW480 and HCT116 human colon cancer cell lines were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultured in DMEM (GIBCO) with 10% FBS in a 5% CO2 incubator. Human dermal lymphatic endothelial cells (HDLEC, Procell) were incubated in an endothelial cell medium (ECM, Sciencell) with 5% FBS. The 293T cell line was obtained from the Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University and cultured in DMEM with 10% FBS in a humidified atmosphere with 5% CO2.
2.4. Stable cell line generation
The psuper‐retro‐puro shTRAF6 recombinant lentivirus (psuper‐TR1 and psuper‐TR2) and the empty psuper‐retro‐puro shRNA lentivirus (psuper) were constructed by our laboratory according to our previous research. 14 The recombinant plasmid or empty vector was transfected with packaging plasmids pIK (Invitrogen) into 293T cells. The opening reading frame (ORF) of the human TRAF6 gene was PCR amplified and inserted into the lentiviral expression vector pCDH‐CMV‐MCS‐EF1‐GFP‐Puro (System Biosciences). The recombinant plasmid pCDH‐3×Flag‐TRAF6 or pCDH‐3×Flag‐TRAF6 124mut and the empty pCDH, lentivirus plasmid pMD2.G, and psPAX2 (Hanbio Biotechnology) were co‐transfected in 293T cells at 90% confluence. 14 Puromycin was used to select cells with a stable expression of psuper‐TR1, psuper‐TR2, psuper, pCDH‐3×Flag‐TRAF6, pCDH‐3×Flag‐TRAF6 124mut and pCDH.
2.5. Western blot analysis
The protein extraction process was carried out on the ice. Western & IP cell lysis buffer (Beyotime) containing 1× phenylmethylsulfonyl fluoride (PMSF; Sangon Biotech) and 1× cocktail (MedChemExpress) was used to lyse cells for 30 min. The lysed cultured cells were then scraped off the surface of the culture dish, and the culture cells were centrifuged at 13 500 rcf for 10 min. The centrifuged cell supernatant was extracted with a BCA Protein Assay Kit (Thermo Scientific), SDS buffer (1×) was added to the supernatant to boil for 10 min, 40 μg protein was added to 10% SDS‐PAGE for electrophoresis and subsequently transferred to a 0.45 μM PVDF membrane (Amersham Hybond, GE Healthcare). The membrane was then immersed in a blocking solution containing 1% BSA (Amresco, Solon, Ohio, USA), incubated in the TRAF6 antibody (AF5376 Affinity 1:1 000 [Affinity Biosciences]), VEGF‐C antibody (DF7011 affinity 1:1 000 [Affinity Biosciences]), rabbit anti‐NF‐κB p65 (#82421:1 000; Cell Signaling Technology), rabbit anti‐phospho‐NF‐κB p65 (#30331:1 000; Cell Signaling Technology), anti‐GAPDH (ab1816021:1 000; Abcam), FLAG antibody(1:1000 Abcam), and HA antibody(1:1 000 Abcam) overnight at 4℃. After washing three times with TBS‐T (0.1% Tween‐20), the membrane was incubated with secondary antibodies of the corresponding antibodies. The membranes were analyzed with enhanced chemiluminescence substrate detection solution (Lulong Biotech).
2.6. Lymphatic tube formation assay
SW480 and HCT116 cells were transfected with TRAF6 and TRAF6 shRNA in a lentivirus, respectively. Cell supernatants were harvested after 48 h in serum‐free DMEM medium. Cell supernatants were added to 10 × 10³ HDLEC/well in a 96‐well plate pre‐coated with Matrigel and serum‐free medium. After 6 h, the lymphatic tube formation was observed with a microscope. Tubes were quantified by the number of branches. Each experiment was repeated three times.
2.7. Dual‐luciferase reporter assay
SW480 and HCT116 colorectal cancer cells were seeded in 24‐well plates. pcDNA3.1‐TRAF6 and pNF‐κB‐Luc, or VEGF‐C promoter were individually transfected or co‐transfected into the cells and then co‐transfected with pRL‐TK reporter vector. The Dual Luciferase Reporter Assay Kit (Vazyme) was used 48 h after transfection to obtained cell lysates, in accordance with the manufacturer’s recommendations. The corresponding luciferase activity was detected with a Dual‐Luciferase Reporter Assay System (Promega) with a luminometer (Orion II Microplate Luminometer, Berthold Detection Systems). Each transfection was carried out in triplicate.
2.8. RNA extraction and reverse transcription real‐time quantitative PCR
RNA was extracted from cells according to the instructions of the TRIzol Reagent (Ambion). Extracted RNA was incubated with RQ1 RNase‐free DNase (Promega) to remove DNA. Agarose gel electrophoresis with ethidium bromide staining was performed to detect the extracted total RNA. The concentration of RNA was determined with a spectrophotometer (Eppendorf). RNA was reverse transcribed into cDNA according to the instruction manual of the RT Reagent Kit (Takara). Quantitative PCR was performed with an SYBR Premix EX Taq kit (Takara) to test TRAF6, VEGF‐C, and β‐actin. All primers were synthesized by BioSun Biotechnology. The expression level of the target gene was calculated as 2−∆∆CT. The expression level of the target gene was normalized to endogenous β‐actin.
2.9. Ubiquitination assay
Immunoprecipitations of ubiquitinated protein were performed with SW480 and HCT116 cells transiently overexpressing TRAF6, or 124mut and HA‐Ub, K63‐Ub, or K48‐Ub. For western blots and immunoprecipitation, cell lysis buffer (Beyotime) containing 1×PMSF and 1× cocktail was used to lyse cells. A BCA Protein Assay Kit (Thermo Scientific) was used for protein quantification; 2 mg protein was placed on 20 μL Anti‐DYKDDDDK Affinity Beads (Pierce) and mixed at 4℃ overnight. After centrifugation, the supernatant was discarded. After washing several times, the remaining beads were added to 2× SDS and boiled for 10 min. TRAF6 ubiquitination was determined by western blotting and HA antibody (Pierce).
2.10. Colony‐forming assay
Cells were seeded onto six‐well plates at a density of 600 cells per well for colony formation. HCT116 and SW480 cells were cultured in an incubator for 9 days and 2 weeks, respectively. The colony cells were visualized with methanol fixing and 1% crystal violet (Beyotime Biotechnology) staining for 10 min, respectively. Colonies of 50 cells were counted. The number of colony cells was calculated using ImageJ (National Institutes of Health). Each experiment was repeated three times.
2.11. Scratch‐wound assay
Next, 2.5 × 106 cells were seeded in six‐well plates until confluence was reached. A scratch was made through the cell layer using a pipette tip. The serum‐free medium was added after washing with PBS three times. Photographs of the wound distance were taken immediately after scratching with the ZEN lite camera (ZEISS). The movement of the cells into the wounded distance was monitored after 48 h. Each experiment was repeated three times.
2.12. Cell migration and invasion assay
A transwell chamber (8 μM, 24‐well format; BD‐Falcon, USA) and Matrigel‐coated transwell chamber (Corning, USA) were inserted into 24 well plates to measure migration and invasion. After being serum starved for 24 h, 10 × 104 cells in 0.5 mL of serum‐free DMEM medium were added in the upper chamber, and 0.7 mL of DMEM containing 15% FBS was added in the lower chamber. After 48 h of incubation, the upper‐surface cells were removed with a cotton swab and the lower‐surface cells were stained with 1% crystal violet for 5 min. Five fields were randomly selected to count and photograph the migrating cells in each well at 100× magnification. Each experiment was repeated three times.
2.13. Mouse studies
Nude mouse experiments were conducted at the Animal Experimental Center of Fujian Medical University and approved by the University Ethics Committee. Orthotopic‐transplantation models and subcutaneous xenograft models of colon cancer were established in female nude mice (BALB/c, aged 5 weeks). Surgical laparotomy was performed by intraperitoneal injection to find the colon of nude mice, then 5 × 106 HCT116‐psuper cells or HCT116‐psuper‐TR2 cells were injected into colorectal submucosa. HCT‐116‐pCDH‐3×Flag‐TRAF6 or HCT116‐pCDH‐3×Flag‐TRAF6 124mut cells were injected into five mice subcutaneously, respectively. Tumors were palpated through the abdomen every 3 days. The nude mice were killed after approximately 6 weeks. Subcutaneous xenograft tumors were removed, photographed, and immediately weighed and measured. The formula for tumor volume measurement is length × width2/2. Orthotopic‐transplantation tumors were soaked in formalin for immunohistochemical analysis.
2.14. H&E staining and analysis
Before sectioning and staining, fresh orthotopic tumors and lymph node tissues were fixed in 10% formalin and embedded in paraffin. The tissue section (4 μm) was deparaffinized in xylene and rehydrated in an ethanol series. A standard protocol 15 was used to perform H&E staining. Image acquisition was performed with the cellSens microscope (Olympus). Images were analyzed with INFINITY ANALYZE software (Lumenera). 16
2.15. Statistical analysis
GraphPad Prism 8 software was used for statistical analyses. The data were expressed as the means ± standard deviation (SD) and analyzed by one‐way ANOVA or Student’s t‐test. Differences with a p‐value <0.05 were considered statistically significant.
3. RESULTS
3.1. TRAF6 expression correlated lymphangiogenesis in patient tumor
A total of 100 clinical samples from colorectal cancer patients and adjacent tissue were analyzed for TRAF6 expression, which was higher in colorectal cancer tissues than adjacent normal tissue (Figure 1A,B). Correspondingly, the number of lymphatic vessels in colorectal cancer tissues was more than that in normal colorectal tissues (Figure 1A,B). The MOD of TRAF6 expression and the LVD in normal colorectal tissues were significantly lower than in colorectal cancer tissues (P < 0.001) (Figure 1C,D). The expression of TRAF6 was analyzed in clinical samples of 100 colorectal cancer patients according to N staging, and it was found that the expression of TRAF6 in N1 and N2 staging colorectal cancer tissues was higher than that of N0 (Figure 1E,F). Thus, the increase of LVD may be related to the expression of TRAF6.
FIGURE 1.
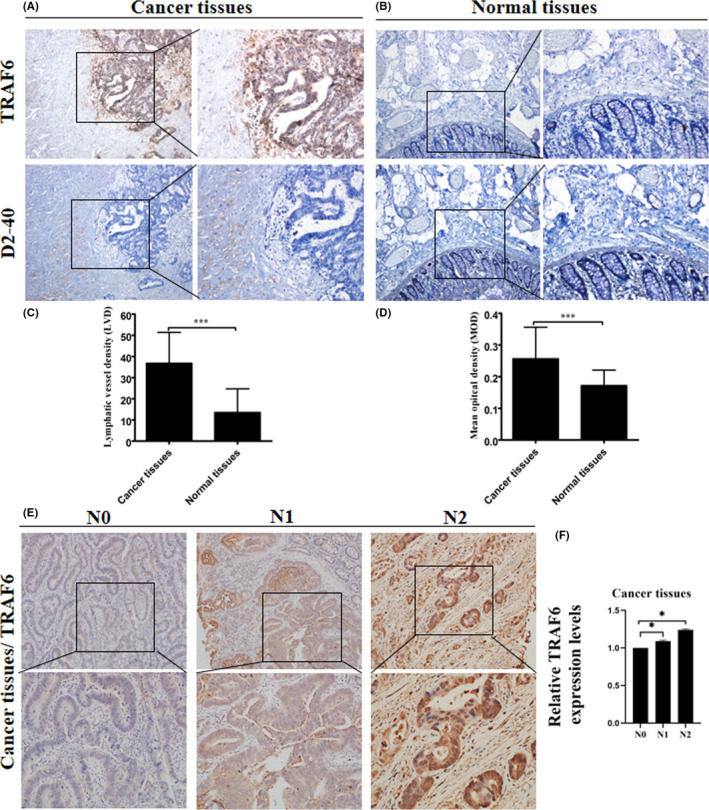
Expression of TRAF6 and lymphatic vessels in colorectal cancer clinical samples. (A, B) Representative immunohistochemical staining images for TRAF6 protein and D2‐40 in colorectal cancer (CRC) and adjacent normal tissues from a tissue array are shown. Magnification, ×40 and ×100. (C) Mean optical density (MOD) showed that TRAF6 expression in colorectal cancer tissues was elevated compared with normal colorectal tissues. (D) The result of LVD showed that the expression of lymphatic vessels in colorectal cancer tissues was elevated compared with normal colorectal tissues. (E) Representative immunohistochemical staining images for TRAF6 protein in CRC tissues from a tissue array were shown. Magnification, ×100 and ×200. (F) The result of immunohistochemical staining showed that the expression of relative TRAF6 expression levels in N1 and N2 stage colorectal cancer tissues was elevated compared with N0. Data are shown as mean ±SD. ***P < 0.001
3.2. TRAF6 promotes HLEC tube formation
Western blot was used to detect the expression effect of TARF6 in overexpression stable CRC cell lines and silent stable CRC cell lines. The expression of TRAF6 protein was significantly increased in the TRAF6 overexpression stable cell line (Figure 2A,C). The expression of TRAF6 protein was significantly reduced in the TRAF6 silent stable cell lines (Figure 2E,G). These results indicated that the stable CRC cell lines of TRAF6 were successfully constructed. To determine the effect of TRAF6 on lymphangiogenesis, the lymphatic tube formation assay was performed. The pCDH‐3×Flag‐TRAF6 group had increased tube numbers compared to the pCDH group (Figure 2B,D). When TRAF6 was knocked down by psuper‐TR1, and psuper‐TR2, the HLEC tube number was significantly decreased (Figure 2F,H). These results showed that TRAF6 promotes lymphangiogenesis.
FIGURE 2.

The role of TRAF6 in HLEC tube formation. (A, C) Western blotting analysis of TRAF6 protein expression in SW480 and HCT116 cells transfected with pCDH and pCDH‐TRAF6, respectively. GAPDH served as a loading control. The relative protein expression levels of TRAF6 in SW480 and HCT116 cells were quantified. (B, D) The lymphatic tube number (40×) of the pCDH‐TRAF6 cells increased in comparison with the pCDH transfected cells (negative control). One representative image from three reproducible experiments is shown. The number of lymphatic tubes is shown in the bar graph. (E, G) Western blot analysis of TRAF6 expression in SW480 and HCT116 cells transfected with psuper, pTR1 (psuper‐shTRAF6‐1), and pTR2 (psuper‐shTRAF6‐2), respectively. GAPDH served as a loading control. The relative protein expression levels of TRAF6 in SW480 and HCT116 cells were quantified. (F, H) The tube number of pTR1 and pTR2 transfected cells was significantly reduced by comparison with psuper cells (negative control). One representative image from three reproducible experiments is shown. The number of lymphatic tubes is shown in the bar graph. Data are shown as mean ±SD. *P < 0.05
3.3. TRAF6 expression affects the NF‐κB‐VEGF‐C signaling pathway
In a previous study, 17 we found that TRAF6 expression affected the growth and progression of colorectal cancer in the NF‐κB signaling pathway. VEGF‐C is a special lymphatic endothelial growth factor that plays an important role in lymphangiogenesis. 18 VEGF‐C is one of the main factors involved in the molecular mechanism of lymphangiogenesis. 19 Western blotting analysis showed that pNF‐κB and VEGF‐C protein levels in pCDH‐3×Flag‐TRAF6 cells increased compared with pCDH cells (Figure 3A,B). pNF‐κB and VEGF‐C protein levels were significantly decreased after TRAF6 was knocked down by TRAF6 shRNA (Figure 3C,D). These results demonstrated that TRAF6 expression affects the NF‐κB‐VEGF‐C signaling pathway.
FIGURE 3.

Effect of TRAF6 expression on NF‐κB, pNF‐κB, and VEGF‐C expression in SW480 and HCT116 cells. (A, B) Western blotting analysis of NF‐κB, pNF‐κB, and VEGF‐C expression in SW480 and HCT116 cells transfected with pCDH and pCDH‐TRAF6, respectively. GAPDH served as a loading control. (C, D) The relative protein expression levels of NF‐κB, pNF‐κB, and VEGF‐C in SW480 and HCT116 cells were quantified. Data are shown as mean ±SD. *P < 0.05; **P < 0.01; ***P < 0.001
3.4. Effect of TRAF6 expression on the NF‐κB‐VEGF‐C signaling pathway
To confirm the effect of TRAF6 expression on NF‐κB and VEGF‐C transcriptional levels, the pNF‐κB‐Luc or VEGF‐C promoter was co‐transfected into CRC cells with different concentrations of pcDNA3.1‐TRAF6 plasmids. 20 Compared with untreated cells, the activity of pNF‐κB‐Luc (Figure 4A,B) and VEGF‐C promoter (Figure 4C,D) in CRC cells increased as the pcDNA3.1‐TRAF6 concentration was increased. The increases in TRAF6, pNF‐κB, and VEGF‐C protein are correlated (Figure 4E–H). This increase was dependent on the pcDNA3.1‐TRAF6 concentration used to transfect the cells. These results indicated that TRAF6 promotes the NF‐κB‐VEGF‐C signaling pathway.
FIGURE 4.
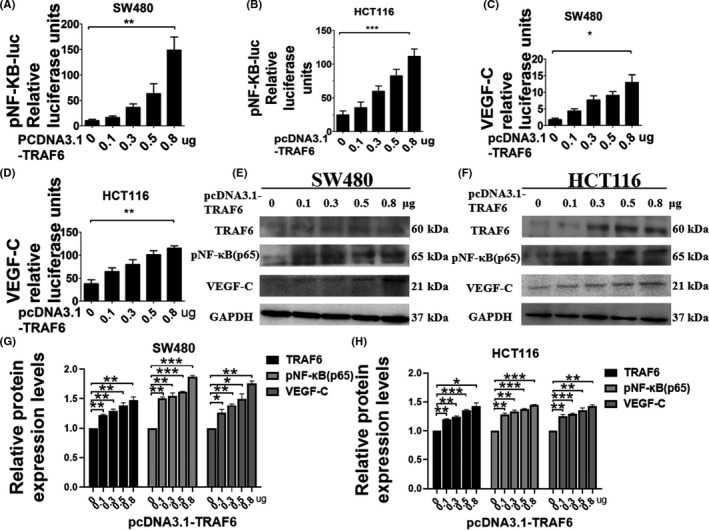
Effects of different concentrations of TRAF6 on NF‐κB and VEGF‐C expression in colorectal cancer (CRC) cells. (A, B) Dual‐luciferase reporter assay analysis of pNF‐κB‐Luc at different concentrations of pcDNA3.1‐TRAF6 plasmids in SW480 and HCT116 cells. The Renilla luciferase express vector pRL‐TK was used for standardization. After transfection for 48 h, the relative activities were measured. (C, D) Dual‐luciferase reporter assay analysis of VEGF‐C promoter at different concentrations of pcDNA3.1‐TRAF6 plasmids in SW480 and HCT116 cells. The Renilla luciferase express vector pRL‐TK was used for standardization. After transfection for 48 h, the relative activities were measured. (E, F) Western blotting analysis of TRAF6, pNF‐κB, and VEGF‐C expression with different concentrations of pcDNA3.1‐TRAF6 plasmids transfected in SW480 and HCT116 cells. GAPDH served as a loading control. (G, H) The relative protein expression levels of TRAF6, pNF‐κB, and VEGF‐C in SW480 and HCT116 cells were quantified. Data are shown as mean ±SD. *P < 0.05; **P < 0.01; ***P < 0.001
3.5. Lipopolysaccharide affects lymphangiogenesis via the TRAF6‐NF‐κB‐VEGF‐C signaling pathway
In a previous study, we found that LPS promoted lymphangiogenesis by increasing the release of VEGF‐C. 9 The expression of TRAF6 increased in SW480 and HCT116 cells after treatment with exogenous LPS (Figure 5A). The number of lymphatic tubes increased in the LPS‐treated cells compared with the untreated cells (Figure 5B). NF‐κB and VEGF‐C transcriptional levels increased after LPS treatment (Figure 5C). In Figure 5D,E, the expression of proteins was detected in different LPS concentrations by western blot. LPS concentration of 1 ug/mL had a significant effect on TRAF6, pNF‐κB, and VEGF‐C (Figure 5D,E). Protein and mRNA expression levels for TRAF6 and VEGF‐C were the highest at 12 h of treatment with LPS (Figure 5F,G). The results indicated that LPS promotes the TRAF6‐NF‐κB‐VEGF‐C signaling pathway.
FIGURE 5.
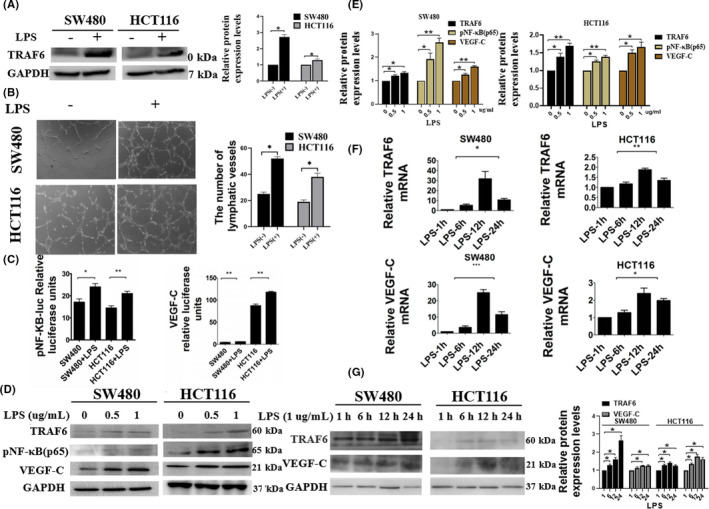
Lipopolysaccharide (LPS) activated the NF‐κB VEGF‐C signaling pathway through TRAF6. (A) Western blot analysis of TRAF6 in colorectal cancer (CRC) cells treated with LPS. GAPDH served as a loading control. The relative protein expression levels of TRAF6 were quantified. (B) Lymphatic tube formation assay analysis for lymphangiogenesis in LPS‐treated SW480 and HCT116 cells. One representative image from three reproducible experiments is shown. The number of lymphatic tubes is shown in the bar graph. (C) Dual‐luciferase reporter assay analysis of pNF‐κB‐Luc and VEGF‐C promoter in LPS‐treated SW480 and HCT116 cells. The Renilla luciferase express vector pRL‐TK was used for standardization. After transfection for 48 h, the relative activities were measured. (D, E) Western blot analysis of TRAF6, pNF‐κB, and VEGF‐C expression with different concentrations of LPS in SW480 and HCT116 cells. GAPDH served as a loading control. The relative protein expression levels of TRAF6, pNF‐κB, and VEGF‐C in SW480 and HCT116 cells were quantified. (F) Quantitative PCR analysis of TRAF6 and VEGF‐C mRNA levels with different times of LPS treatment in SW480 and HCT116 cells. (G) Western blot analysis of TRAF6 and VEGF‐C expression with different times of LPS treatment in SW480 and HCT116 cells. GAPDH served as a loading control. Data are shown as mean ±SD. *P < 0.05; **P < 0.01; ***P < 0.001
3.6. TRAF6 affects the LPS‐NF‐κB‐VEGF‐C signaling pathway via ubiquitination
Tumor necrosis factor receptor‐associated factor‐6 is a ubiquitin‐ligase. The K48‐linked polyubiquitin chain is associated with proteasome degradation, while the K63‐linked polyubiquitin chain is associated with cytokine signaling events. 21 To understand which type of ubiquitination occurs on TRAF6, the ubiquitination assay was performed. The biquitination assay revealed that TRAF6 was ubiquitinated via K63‐linked chains but not K48‐linked chains (Figure 6A,). As is evident from Figure 6C, compared with the untreated group, the K63‐linked ubiquitination level of TRAF6 increased in the LPS‐treated group. Western blot analysis showed that the expression of TRAF6, pNF‐κB, and VEGF‐C proteins enhanced in the LPS‐treated group compared with the untreated group (Figure 6C,D). These data showed that TRAF6 activates the LPS‐NF‐κB‐VEGF‐C signaling pathway via ubiquitination.
FIGURE 6.
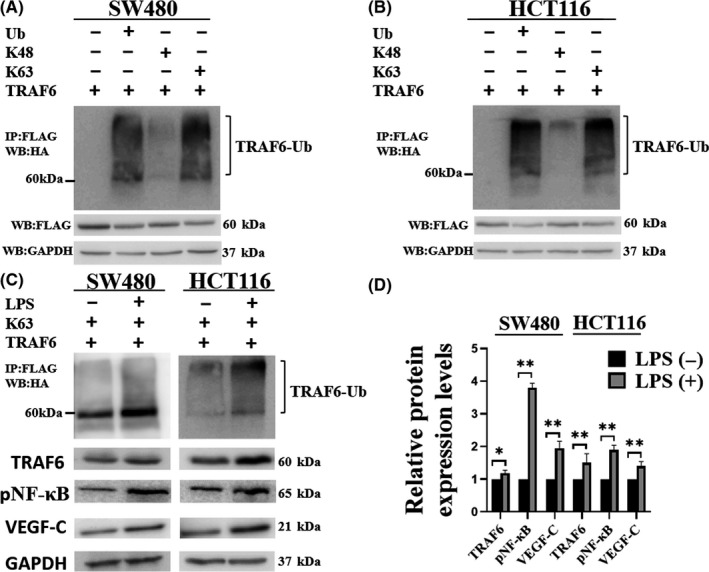
Lipopolysaccharide (LPS) promoted K63‐linked ubiquitination of TRAF6. (A, B) To determine the TRAF6‐mediated ubiquitination type, TRAF6 constructs were transfected into colorectal cancer (CRC) cells with various ubiquitin plasmids. Ubiquitinated TRAF6 was detected with the anti‐HA antibody from immunoprecipitated TRAF6 protein. (C, D) To confirm the effect of LPS on TRAF6 ubiquitination, CRC cells were transiently co‐transfected with pcDNA3.1–3×flag‐TRAF6 and HA‐K63 as indicated. At 36 h post‐transfection, the cells were incubated with LPS for 12 h and then subjected to ubiquitination assay and western blot. Data are shown as mean ±SD. *P < 0.05; **P < 0.01
3.7. 124mut inhibits TRAF6 ubiquitination and the NF‐κB‐VEGF‐C signaling pathway
In our previous study, we found that 124mut, which is a mutant plasmid of TRAF6 at the 124th ubiquitin site, could significantly inhibit the NF‐κB transcriptional level. We investigated whether the 124th site is the key site for TRAF6 ubiquitination. After the mutation of the 124th ubiquitin site of TRAF6, the ubiquitination level at the HA‐Ub and K63‐Ub sites significantly decreased but not at the K48‐Ub site (Figure 7A,B,C). The results indicated that the 124th ubiquitin site of TRAF6 plays an important role in its ubiquitination.
FIGURE 7.

Effect of 124mut on TRAF6 ubiquitination, pNF‐κB, and VEGF‐C expression. (A) Schematic diagram of ubiquitin. (K: lysine; R: arginine). (B, C) To detect the effect of the 124th ubiquitination site mutation on TRAF6 ubiquitination, TRAF6 or TRAF6 124mut construct were transfected into colorectal cancer (CRC) cells with various ubiquitin plasmids, respectively. Ubiquitinated TRAF6 was detected with the anti‐HA antibody from immunoprecipitated TRAF6 protein. (D, E) Western blot analysis of pNF‐κB and VEGF‐C protein levels in SW480 cells after transfection with pCDH‐3×Flag‐TRAF6 or pCDH‐3×Flag‐TRAF6 124mut plasmids. GAPDH served as a loading control. The relative protein expression levels of TRAF6, pNF‐κB, and VEGF‐C in SW480 and HCT116 cells were quantified. Data are shown as mean ± SD. *P < 0.05; **P < 0.01
When the 124th ubiquitin site of TRAF6 was mutated, the expression of pNF‐κB and VEGF‐C proteins significantly decreased (Figure 7D,E). The results further indicated that 124mut inhibits the NF‐κB‐VEGF‐C signaling pathway.
3.8. 124mut inhibits colorectal cancer cell proliferation, migration, and invasion in vitro and tumor growth in vivo
HCT116 or SW480 cells stably overexpressing TRAF6 or 124mut were used to perform the colony‐forming assay (Figure 8A), scratch‐wound assay (Figure 8B,C), migration assay (Figure 8D), and invasion assay (Figure 8E). The proliferation, migration, and invasion potential of CRC cells were significantly reduced after mutation at the 124th site of TARF6. Subcutaneous xenograft models were used to detect the effect of TRAF6 124mut on tumor growth. We subcutaneously injected cell lines of HCT‐116‐pCDH‐3×Flag‐TRAF6 or HCT116‐pCDH‐3×Flag‐TRAF6 124mut into the nude mice. The sizes and weights of HCT‐116‐pCDH‐3×Flag‐TRAF6 tumors were twofold greater than the HCT116‐pCDH‐3×Flag‐TRAF6 124mut tumors (Figure 8F). These results indicated that the 124th ubiquitination site of TRAF6 is the vital site for its function.
FIGURE 8.

TRAF6 124mut inhibits proliferation, migration, and invasion of SW480 and HCT116 cells. (A) Colony‐forming assays were performed to determine the proliferative ability of SW480 and HCT116 cells transfected with pCDH‐3×Flag‐TRAF6 or pCDH‐3×Flag‐TRAF6 124mut plasmids. One representative image from three reproducible experiments is shown. The number of colonies is shown in the bar graph. (B, C, D, E) Scratch‐wound assay (B, C), migration assay (D), and invasion assay (E) were used to detect the metastatic potential of HCT116 and SW480 cells transfected with pCDH‐3×Flag‐TRAF6 or pCDH‐3×Flag‐TRAF6 124mut plasmids, respectively. The number of migrated and invaded cells were counted from five different fields under a microscope. Magnification, ×100. (F) Each of 5×106 HCT116‐pCDH‐3×Flag‐TRAF6 or HCT116‐pCDH‐3×Flag‐TRAF6 124mut cells were injected into nude mice. After 6 weeks, the mice were killed, the tumors were weighed, and the volume was calculated (n = 5). G Schematic diagram of the mechanism of TRAF6 regulating the LPS‐NF‐κB‐VEGF‐C signaling pathway through ubiquitination in colorectal cancer. Data are shown as mean ±SD. *P < 0.05; **P < 0.01; ***P < 0.001
3.9. TRAF6 knockdown inhibits tumor growth, mesenteric lymph node metastasis, TRAF6 expression, and lymphangiogenesis in mouse models
We used HCT116 cells transfected with psuper or psuper‐TR2 to establish orthotopic models of colon cancer in nude mice. Tumor growth was inhibited in the psuper‐TR2 group compared with the control group. Similarly, the extent of mesenteric lymph nodes metastasis was also reduced in the psuper‐TR2 group. H&E staining showed that the cancer‐cell density of the psuper‐TR2 group was lower than that of the psuper group. In the psuper‐TR2 group, the degree of lymph node destruction was significantly lower compared with the control group (Fig. S1A,B). TRAF6 expression levels and lymphangiogenesis were markedly inhibited in the psuper‐TR2 group compared with the control group, as seen with immunohistochemistry (Fig. S1C,D). TRAF6 expression and LVD in the psuper‐TR2 group were lower than the psuper group (Fig. S1E,F).
4. DISCUSSION
Tumor necrosis factor receptor‐associated factor‐6 is not only an intracellular signal transducer but also a variety of extracellular signal mediators. 22 TRAF6 was upregulated in several malignant tumors. 17 , 23 , 24 , 25 Our previous study showed that high expression of TRAF6 was associated with lymph node metastasis. 17 In the present study, we also found that TRAF6 was highly expressed in colorectal cancer tissues in patients compared to adjacent normal tissue. The LVD was also increased in colorectal cancer tissues. The lymphatic tube formation assay analysis showed that overexpression TRAF6 promoted lymphangiogenesis and TRAF6 knockdown inhibited lymphangiogenesis. HCT116 tumor growth and lymph node metastasis were reduced after TRAF6 was knocked down by shRNA. TRAF6 expression levels and LVD were significantly reduced by the psuper‐TR2. The results showed that TRAF6 affected lymphangiogenesis in vitro as well as in vivo.
Some research showed that overexpression of TRAF6 could activate NF‐κB and AP‐1 and play a vital role in NF‐κB and MAPK/AP‐1 signaling pathways. 26 , 27 Meng et al. reported that TRAF6 regulated the carcinogenesis of pancreatic ductal adenocarcinoma (PDAC) cells through the NF‐κB signaling pathway. 28 VEGF‐C is the only special lymphatic endothelial growth factor discovered so far. 29 VEGF‐C is also a central regulator of angiogenesis. 30 In the present study, we demonstrated that TRAF6 could regulate the expression levels of pNF‐κB and VEGF‐C protein in SW480 and HCT116 cells, which suggested that TRAF6 could regulate lymphangiogenesis through the NF‐κB‐VEGF‐C signaling pathway. With the increase of TRAF6 expression, transcription and translation levels of NF‐κB and VEGF‐C were enhanced in CRC cells. The results indicated that TRAF6 activated the NF‐κB‐VEGF‐C signaling pathway.
Lipopolysaccharide is the main component of the outer membrane of gram‐negative bacteria, which is composed of lipids and polysaccharides. LPS often participates in the process of signaling pathways through specific binding of TLR4. 31 Zhang et al. reported that LPS upregulated VEGF‐C by activating the TLR4‐MyD88‐NF‐κB signaling pathway. 32 Our previous study 9 showed that LPS increased the release of VEGF‐C through the TLR4‐NF‐κB/JNK pathway in colorectal cancer. LPS was associated with lymphatic metastasis. Consistent with the research of Pidgeon et al., we found that LPS promoted lymphangiogenesis in vitro using the tube formation assay. In LPS‐treated CRC cells, the activity of pNF‐κB‐Luc and VEGF‐C promoter significantly increased. The increase of TRAF6, pNF‐κB, and VEGF‐C protein expression levels was dependent on the LPS concentration used to treat SW480 and HCT116 cells. After treatment with LPS for 12 h, the effect of LPS on the mRNA and protein expression levels of TRAF6 and VEGF‐C was the most obvious. The results suggested that LPS affected lymphangiogenesis through the TRAF6‐NF‐κB‐VEGF‐C signaling pathway.
Zhang et al. reported that in the TRAF6 pathway, ubiquitination of TRAF6 played a vital role in triggering the initial activation step. IKK was activated by TRAF6 through the K63‐linked polyUb chain rather than proteasome degradation. 33 Some proteins involved in the NF‐κB signaling pathway underwent ubiquitination following ligand binding, such as TNF‐α. 34 In the present study, TRAF6 was ubiquitinated mainly at K63 in CRC cells. LPS promoted the ubiquitination of TRAF6 with K63‐linked chains. Meanwhile, the expression levels of TRAF6, pNF‐κB, and VEGF‐C proteins increased under the same treatment conditions. LPS also promoted lymphangiogenesis, suggesting that these genes might be involved in lymphangiogenesis.
In previous studies, we found that the 124th ubiquitination site of TRAF6 was the most important ubiquitination site affecting the activity of NF‐κB in downstream signaling pathways. The ubiquitination of TRAF6 and Ub, or K63 linkage, was lower after the 124th amino acid of TRAF6 was mutated. When the 124th ubiquitination site of TRAF6 was mutated, the expression levels of pNF‐κB and VEGF‐C proteins decreased. We hypothesize that the 124th ubiquitination site of TRAF6 is a key site for TRAF6 to participate in the LPS‐NF‐κB‐VEGF‐C signaling pathway. It was reported that TRAF6 promoted tumorigenesis by promoting tumor proliferation and invasion. 35 The results are consistent with our experimental results. We found that CRC cell proliferation, migration, and invasion were decreased by mutation at the 124th site of TRAF6. Moreover, TRAF6 124mut significantly inhibited tumor growth compared with overexpression of TRAF6. The results indicated that the 124th site of TRAF6 was its functional ubiquitination site.
In summary, the present study showed that the LVD increases in colorectal cancer with high TRAF6 expression. Ubiquitination of TRAF6 affects lymphangiogenesis in colorectal cancer by activating LPS‐NF‐κB‐VEGF‐C signaling (Figure 8G). The 124th ubiquitination site of TRAF6 not only affected the activation of the LPS‐NF‐κB‐VEGF‐C signaling pathway but also affected the proliferation, migration, and invasion of CRC cells in vitro and tumor growth in vivo. The present study indicates that specific ubiquitination of TRAF6 plays a critical role in lymphatic metastasis of colorectal cancer and may serve as a potential therapeutic target.
DISCLOSURE
The authors declare no conflicts of interest regarding this study.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (No. 81702424 and 81872364), the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2020Y9017), the Fujian Provincial Health Technology Project (No. 2020CXA032), and the Startup Fund for Scientific Research of Fujian Medical University (No. 2020QH2035).
Guangwei Z, Zhibin C, Qin W, et al. TRAF6 regulates the signaling pathway influencing colorectal cancer function through ubiquitination mechanisms. Cancer Sci. 2022;113:1393–1405. doi: 10.1111/cas.15302
Funding information
Joint Funds for the Innovation of Science and Technology, Fujian Province, (Grant/Award Number: No. 2020Y9017). National Natural Science Foundation of China (Grant/Award Number: No. 81702424, No. 81872364). Startup Fund for Scientific Research of Fujian Medical University, (Grant/Award Number: No. 2020QH2035). Fujian Provincial Health Technology Project (Grant/Award Number: No. 2020CXA032)
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109‐3116. [DOI] [PubMed] [Google Scholar]
- 3. Kraus S, Nabiochtchikov I, Shapira S, Arber N. Recent advances in personalized colorectal cancer research. Cancer Lett. 2014;347:15‐21. [DOI] [PubMed] [Google Scholar]
- 4. Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922‐934. [DOI] [PubMed] [Google Scholar]
- 5. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573‐583. [DOI] [PubMed] [Google Scholar]
- 6. Hirakawa S, Brown LF, Kodama S, et al. VEGF‐C‐induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2006;109:1010‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF‐A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu G, Huang Q, Zheng W, et al. LPS upregulated VEGFR‐3 expression promote migration and invasion in colorectal cancer via a mechanism of increased NF‐kappaB binding to the promoter of VEGFR‐3. Cell Physiol Biochem. 2016;39:1665‐1678. [DOI] [PubMed] [Google Scholar]
- 9. Zhu G, Huang Q, Huang Y, et al. Lipopolysaccharide increases the release of VEGF‐C that enhances cell motility and promotes lymphangiogenesis and lymphatic metastasis through the TLR4‐ NF‐kappaB/JNK pathways in colorectal cancer. Oncotarget. 2016;7:73711‐73724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lomaga MA, Yeh W‐C, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin‐1, CD40, and LPS signaling. Genes Dev. 1999;13:1015‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen P. The TLR and IL‐1 signalling network at a glance. J Cell Sci. 2014;127:2383‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou X, Li Y, Ding J, et al. Down‐regulation of tumor necrosis factor‐associated factor 6 is associated with progression of acute pancreatitis complicating lung injury in mice. Tohoku J Exp Med. 2009;217:279‐285. [DOI] [PubMed] [Google Scholar]
- 13. Han C, Yan P, He T, et al. PHLDA1 promotes microglia‐mediated neuroinflammation via regulating K63‐linked ubiquitination of TRAF6. Brain Behav Immun. 2020;88:640‐653. [DOI] [PubMed] [Google Scholar]
- 14. Ye J, Xu J, Li Y, et al. DDAH1 mediates gastric cancer cell invasion and metastasis via Wnt/beta‐catenin signaling pathway. Mol Oncol. 2017;11:1208‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oshiro H, Kiyuna T, Tome Y, et al. Detection of metastasis in a patient‐derived orthotopic xenograft (PDOX) model of undifferentiated pleomorphic sarcoma with red fluorescent protein. Anticancer Res. 2019;39:81‐85. [DOI] [PubMed] [Google Scholar]
- 16. Igarashi K, Kawaguchi K, Murakami T, et al. High efficacy of pazopanib on an undifferentiated spindle‐cell sarcoma resistant to first‐line therapy is identified with a patient‐derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2739‐2743. [DOI] [PubMed] [Google Scholar]
- 17. Zhu G, Cheng Z, Huang Y, et al. TRAF6 promotes the progression and growth of colorectal cancer through nuclear shuttle regulation NF‐kB/c‐jun signaling pathway. Life Sci. 2019;235:116831. [DOI] [PubMed] [Google Scholar]
- 18. Neuchrist C, Erovic BM, Handisurya A, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck. 2003;25:464‐474. [DOI] [PubMed] [Google Scholar]
- 19. Saharinen P, Petrova TV. Molecular regulation of lymphangiogenesis. Ann N Y Acad Sci. 2004;1014:76‐87. [DOI] [PubMed] [Google Scholar]
- 20. Zhu G, Lin C, Cheng Z, et al. TRAF6‐mediated inflammatory cytokines secretion in LPS‐induced colorectal cancer cells is regulated by miR‐140. Cancer Genomics Proteomics. 2020;17:23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim HY, Kim YM, Hong S. DNAJB9 suppresses the metastasis of triple‐negative breast cancer by promoting FBXO45‐mediated degradation of ZEB1. Cell Death Dis. 2021;12:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin‐1. Nature. 1996;383:443‐446. [DOI] [PubMed] [Google Scholar]
- 23. Rong Y, Wang D, Wu W, et al. TRAF6 is over‐expressed in pancreatic cancer and promotes the tumorigenicity of pancreatic cancer cells. Med Oncol. 2014;31:251‐260. [DOI] [PubMed] [Google Scholar]
- 24. Sun H, Li XB, Li F, et al. TRAF6 is upregulated in colon cancer and promotes proliferation of colon cancer cells. Int J Biochem Cell Biol. 2014;53:195‐201. [DOI] [PubMed] [Google Scholar]
- 25. Han F, Zhang L, Qiu W, Yi X. TRAF6 promotes the invasion and metastasis and predicts a poor prognosis in gastric cancer. Pathol Res Pract. 2016;212:31‐37. [DOI] [PubMed] [Google Scholar]
- 26. Song HY, Regnier CH, Kirschning CJ. Tumor necrosis factor (TNF)‐mediated kinase cascades: bifurcation of Nuclear Factor‐κB and c‐jun N‐terminal kinase (JNK/SAPK) pathways at TNF receptor‐associated factor2. Proc Natl Acad Sci USA. 1997;94:9792‐9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jhj A, Hk A, Jhca B. Molecular cloning and functional characterization of TRAF6 and TAK1 in rainbow trout, Oncorhynchus mykiss ‐ ScienceDirect. Fish Shellfish Immunol. 2019;84:927‐936. [DOI] [PubMed] [Google Scholar]
- 28. Meng Q, Liang C, Hua J, et al. A miR‐146a‐5p/TRAF6/NF‐kB p65 axis regulates pancreatic cancer chemoresistance: functional validation and clinical significance. Theranostics. 2020;10:3967‐3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breiteneder‐Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9‐22. [PubMed] [Google Scholar]
- 31. Shuyi Y, Feng W, Jing T, et al. Human beta‐defensin‐3 (hBD‐3) upregulated by LPS via epidermal growth factor receptor (EGFR) signaling pathways to enhance lymphatic invasion of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:616‐625. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Lu Y, Ma L, et al. Activation of vascular endothelial growth factor receptor‐3 in macrophages restrains TLR4‐NF‐kappaB signaling and protects against endotoxin shock. Immunity. 2014;40:501‐514. [DOI] [PubMed] [Google Scholar]
- 33. Deng L, Wang C, Spencer E, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin‐conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351‐361. [DOI] [PubMed] [Google Scholar]
- 34. Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 Bind to NEMO (IKKγ) upon receptor stimulation. Immunity. 2000;12:301‐311. [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Wu L, Xiao T, et al. TRAF6 regulates EGF‐induced cell transformation and cSCC malignant phenotype through CD147/EGFR. Oncogenesis. 2018;7:7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


