FIGURE 1.
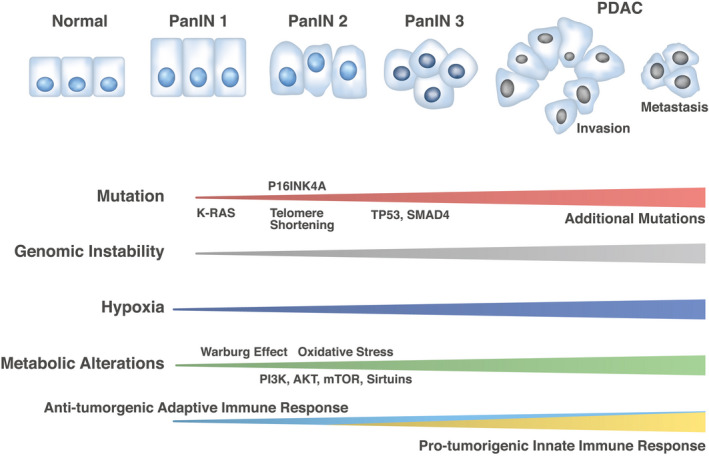
Genomic and metabolic alterations in pancreatic ductal adenocarcinoma (PDAC) development. The schema represents the development of PDAC from pancreatic intraepithelial neoplasia (PanIN) 1, 2, and 3 in the precancer stages. Clinical sequencing results indicate the mutation frequencies of KRAS (99%), TP53 (85%), and SMAD4 (55%) and telomere shortening (91%), although numerous additional mutations occur during the metastatic process. 59 Tissue abnormality has been associated with hypoxia and the Warburg effect at early and advanced stages of pancreatic carcinogenesis, 60 , 61 but recent studies have shown that cancer metabolism alterations such as phosphatidylinositol‐3 kinase (PI3K), AKT, mammalian target of rapamycin (mTOR), and sirtuins are supposed to be involved at least from PanIN 2 stages and can elicit oxidative stress and damage responses associated with genomic instability. 62 , 63 Such alterations may induce further mutations in advanced stages of PDAC. Antitumorigenic adaptive immune response may be involved in early stages of pancreatic carcinogenesis, whereas protumorigenic innate immune response may be involved in advanced stages of PDAC. 64 Oxidative stress controls the function of inflammatory cell types and mechanisms underlying genomic instability in PDAC. 65 As the effect of genomic instability, numerous neoantigens can be targets of immune cells 64 , 65
