Figure 1: Genes associated with pubertal timing across the lifespan.
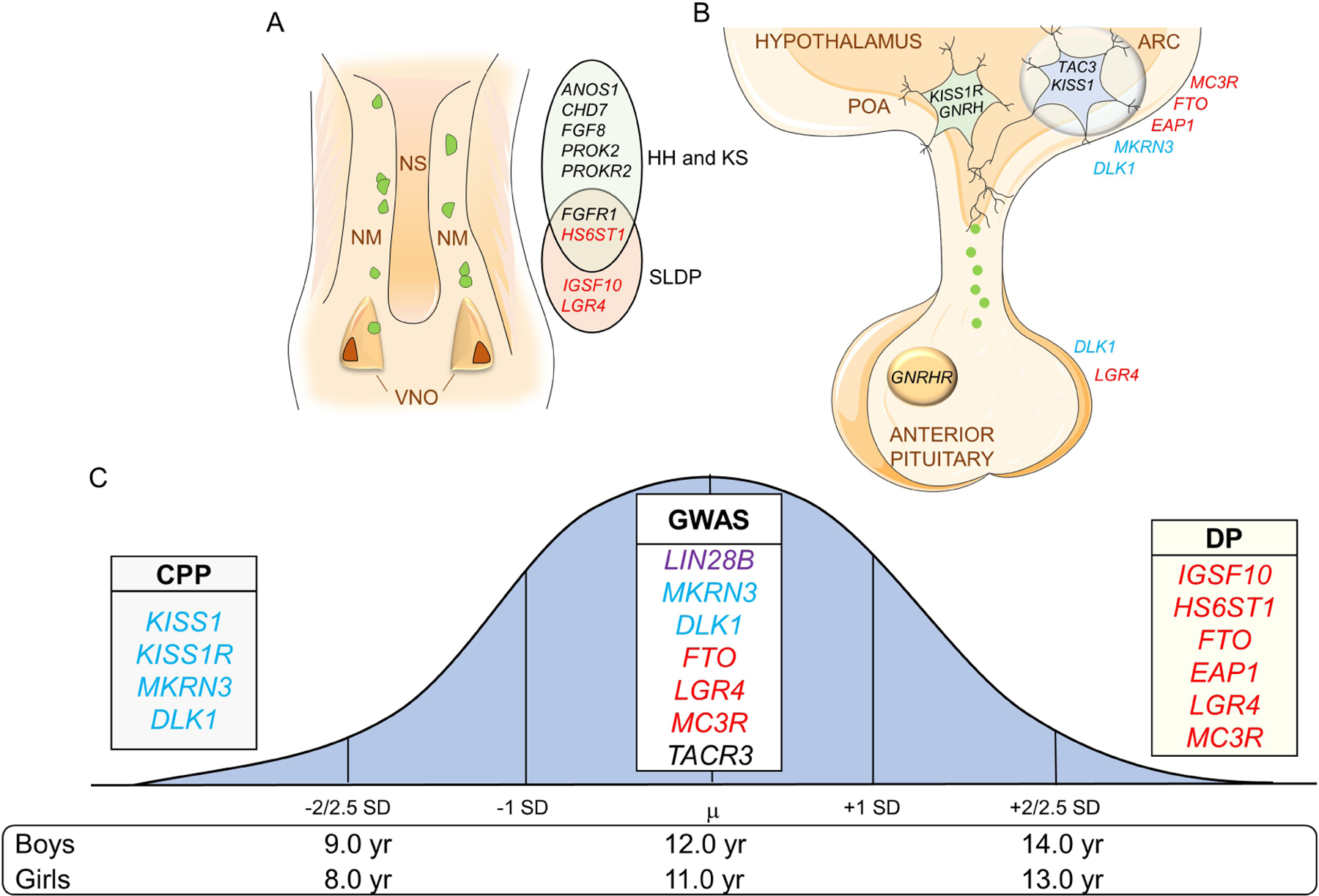
Genes in blue are associated with CPP, in red with DP, and genes in black are associated with HH and/or KS. Variants in LIN28B, in purple, have only been associated with pubertal onset in the normal population. A. Representation of the nasal placode at embryonic life and key genes important for GnRH neuronal migration. GnRH neurons (in green) will migrate alongside olfactory axons from the VNO to the forebrain at the level of the olfactory bulbs (not shown). Mutations in these genes can cause dysregulation in GnRH neuronal migration resulting in delayed puberty and/or HH-KS. B. Figure depicting the hypothalamic and pituitary regions involved in the central regulation of the reproductive axis and key genes controlling GnRH secretion. Kisspeptin-Neurokinin B-Dynorphin (KNDy) neuron is shown in blue in the ARC communicating with GnRH neuron (in green) in the POA. C. Normal distribution of the timing of puberty in the general population with the mean (µ) age of onset. ±2 to 2.5 standard deviation (SD) values indicate precocious or delayed onset of puberty. List of some of the important genes identified in genome-wide association studies (GWAS) and in patient-cohort based studies associated with timing of pubertal onset. VNO: vomeronasal organs; NM: nasal mesenchyme; NS: nasal septum; HH: hypogonadotropic hypogonadism; KS: Kallmann syndrome; SLDP: self-limited delayed puberty. ARC: Arcuate nucleus, POA: preoptic area, CPP: central precocious puberty, DP: delayed puberty.
