Figure 2. Schematic representation of MKRN3 protein structure and 59 mutations identified in patients with CPP.
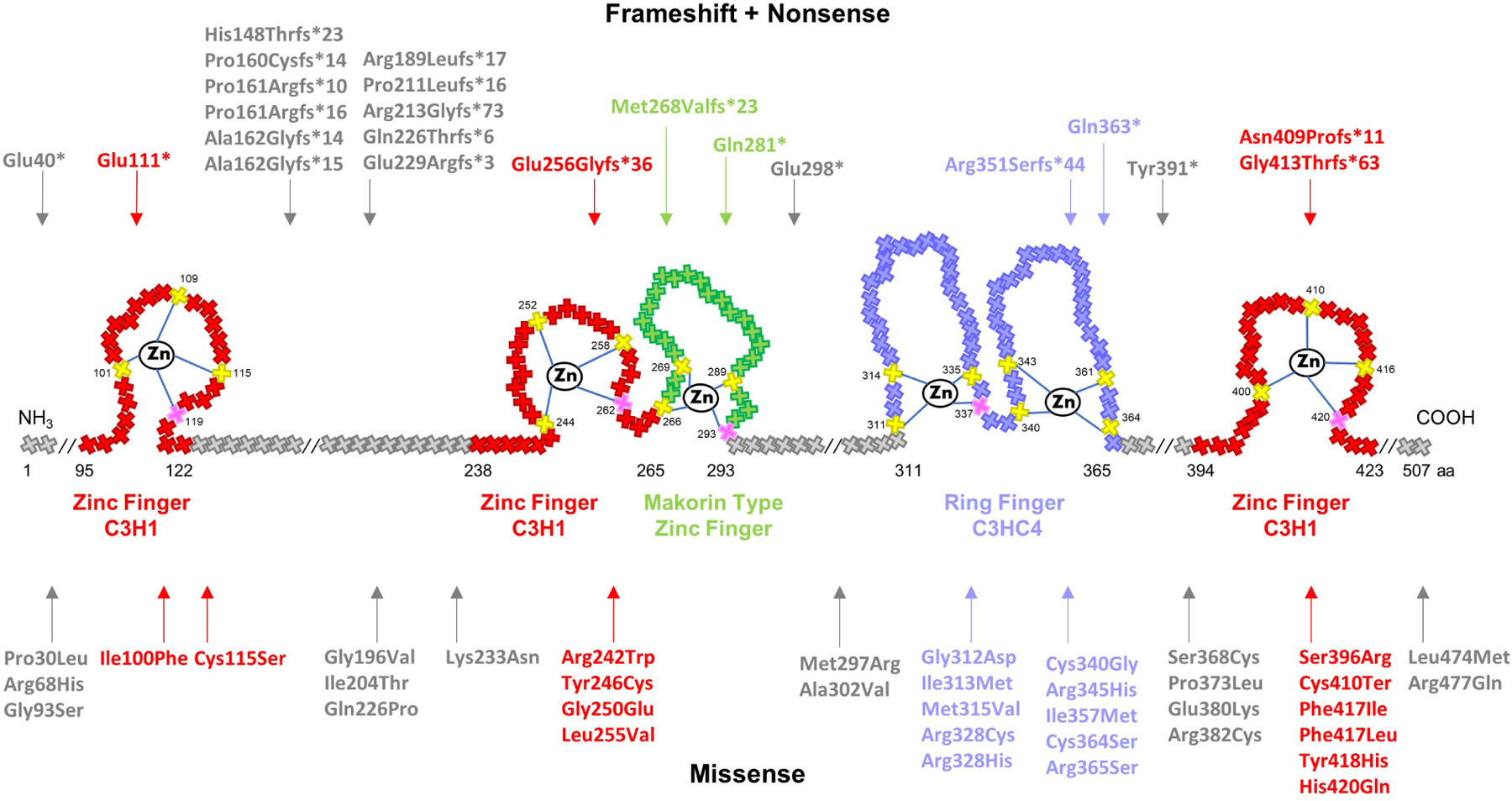
Crosses represent individual amino acids, and corresponding numbers indicate amino acid position. Yellow and pink crosses represent key cysteine and histidine amino acids, respectively, necessary for zinc ion interaction. RING finger C3HC4, in purple, is a protein binding domain responsible for ubiquitin ligase activity. Zinc Finger C3H1, in red, are RNA binding domains. Makorin type Zinc finger, in green, is a specific Cys–His domain identified in the proteins of the makorin family. Notably, 15 mutations (27%) were detected between the first two C3H1 domains, 11 of which are frameshift. Mutations tend to also cluster within the C3HC4 RING finger domain (20%)- the vast majority of which are missense.
