Abstract
Human cytomegalovirus (HCMV) infection does not generally cause problems in the immunocompetent adult but can result in severe clinical disease in the fetus, neonate, and immunocompromised host. Ganciclovir (GCV), the agent currently used to treat most HCMV infections, has resulted in much therapeutic success; however, efficacy remains suboptimal. Therefore, there is still a need to develop new compounds for use against HCMV infections. In the present study, several Z- and E-series methylenecyclopropane analogues and their phosphoroalaninate prodrugs were tested initially for activity against HCMV, strain AD169, and murine cytomegalovirus (MCMV) in vitro. Many were found to exhibit efficacy comparable to that of GCV against HCMV in plaque assays and were active against MCMV as well. The compounds were also tested for efficacy against herpes simplex virus types 1 and 2, varicella-zoster virus, and Epstein-Barr virus, and some had levels of activity that were comparable to that of acyclovir. In addition, the compounds synguanol (QYL-438) and 2-amino-6-cyclopropylamino analogue (QYL-769) were chosen for further evaluation and were found to be effective against additional laboratory and clinical isolates of HCMV and GCV-resistant isolates. QYL-438 and QYL-769 were found to be nontoxic in human and mouse fibroblasts and were considerably less toxic than GCV in granulocyte macrophage CFUs and erythroid burst-forming units. These results provide evidence for the high activity of some of these methylenecyclopropane analogues against various herpesviruses, particularly HCMV, in tissue culture and suggest that further evaluation is warranted to determine their potential for use in future clinical studies.
Human cytomegalovirus (HCMV) infections are the most common cause of congenital viral infections, occurring in 1 to 2% of all live births (28). This β-herpesvirus is typically benign yet can result in a multitude of clinical syndromes, particularly in the immunocompromised host (9, 13). Although HCMV infects approximately 40 to 80% of the U.S. population, the immunocompetent individual rarely manifests overt symptoms (5, 9, 13). The fetus, neonate, and immunocompromised patient are most vulnerable to severe disorders, such as interstitial pneumonia, retardation, hearing loss, microcephaly, and a mononucleosis-like syndrome, some of which can be fatal (8, 16, 24). Acquired or reactivated virus may occur in up to 80 to 90% of renal transplant patients, often resulting in devastating disease or even death (11). The rapid increase in the number of cases of HCMV infections resulting from organ and bone marrow transplantation, cancer chemotherapy, and AIDS has prompted a need to develop more efficacious and less toxic therapeutic agents (1, 22).
Several antiviral compounds have demonstrated efficacy against HCMV, such as ganciclovir (GCV), foscarnet (PFA), acyclovir (ACV), and cidofovir (5, 14). GCV has previously demonstrated efficacy both orally and parenterally in murine cytomegalovirus (MCMV)-infected mice (4, 8, 10, 12, 25) and has been highly effective in humans as well. Although the treatment of CMV retinitis, gastrointestinal disease, and pneumonia is very effective with GCV, relapses are common once treatment is terminated, and long-term drug therapy must be sustained in order to maintain antiviral activity (2, 9). In addition, neutropenia and thrombocytopenia may occur as major side effects, and resistant isolates may develop as well (3, 14, 15).
The recent development of nucleoside analogues with a Z- or E-methylenecyclopropane moiety has led to the evaluation of these compounds as possible antiviral agents. As reported previously, the replacement of the ribofuranose moiety of unsaturated acyclic nucleoside analogues by a rigid allenic residue (adenallene and cytallene) has demonstrated significant anti-human immunodeficiency virus efficacy (6). This development led to the synthesis of a new group of compounds which possess a methylenecyclopropane system substitution in place of the allenic residue. In addition, lipophilic phosphate prodrugs of methylenecyclopropane analogues have exhibited antiviral activity in past studies (21, 23, 29). Thus, compounds with potent antiviral activity against a broader range of viruses, such as several of the herpesviruses, were developed. Many of these nucleoside analogues have been evaluated for antiviral activity and have demonstrated in vitro and in vivo activities against HCMV and MCMV as previously reported (18, 20, 23).
The purpose of the following studies was to further evaluate the antiviral activity of several methylenecyclopropane analogues and phosphoroalaninate prodrugs for their efficacy against various herpesviruses, particularly the cytomegaloviruses, in vitro. The compounds were compared to GCV against HCMV and MCMV, and to ACV against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2, respectively), varicella-zoster virus (VZV), and Epstein-Barr virus (EBV). In addition, all compounds were evaluated for cellular toxicity in fibroblast, lymphoblastic, or bone marrow precursor cells.
MATERIALS AND METHODS
Media and virus strains.
The medium utilized was Earle's minimal essential medium (MEM) (Mediatech, Inc., Herndon, Va.) containing Earle's balanced salt solution supplemented with either 2 or 10% fetal bovine serum, 100 U of penicillin per ml, 25 μg of gentamicin per ml, and 2 mM l-glutamine.
The HCMV strain used for determining activity initially was AD169. Additional clinical and GCV-resistant HCMV strains were provided by Karen Biron of Glaxo Wellcome (Research Triangle Park, N.C.). The GCV-resistant isolates all were UL97 mutants except 759RD100, which had a mutation in UL97 and in the DNA polymerase. Virus strain E-377 was utilized for HSV-1, and strain MS was utilized for HSV-2. The strains used for EBV and VZV were P3HR-1 and Ellen, respectively.
Cell cultures.
Human foreskins were obtained from the University of Alabama at Birmingham Hospital and the human foreskin fibroblasts (HFF) were prepared for use in the HCMV, HSV-1, HSV-2, and VZV assays and for cytotoxicity and cell proliferation assays (7). Mouse embryo fibroblasts (MEF) were prepared from mouse embryos (age, 14 to 16 days) as reported previously (7) for use in MCMV assays. Daudi cells were used in assays to determine drug efficacy against EBV and in lymphoblastic cell proliferation assays for toxicity. Granulocyte macrophage CFU (CFU-GM) and erythroid burst-forming units (BFU-E) were used for bone marrow progenitor cell toxicity evaluation (26).
Antiviral drugs.
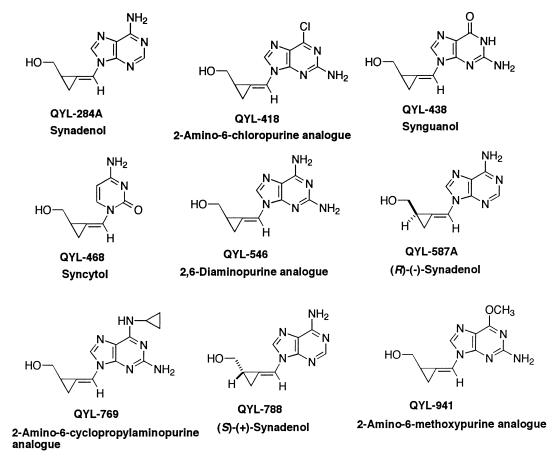
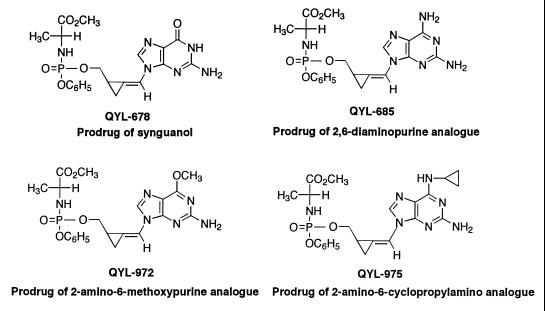
The methylenecyclopropane analogues and prodrugs evaluated were as follows: QYL-284A (synadenol), QYL-788 [S-(+)-synadenol], QYL-587A [R-(−)-synadenol], QYL-468 (syncytol), QYL-438 (synguanol), QYL-678 (prodrug of synguanol), QYL-769 (2-amino-6-cyclopropylamino analogue), QYL-975 (prodrug of 2-amino-6-cyclopropylamino analogue), QYL-546 (2,6-diaminopurine analogue), QYL-685 (prodrug of 2,6-diaminopurine analogue), QYL-418 (2-amino-6-chloropurine analogue), QYL-941 (2-amino-6-methoxypurine analogue), and QYL-972 (prodrug of 2-amino-6-methoxypurine analogue). The synthesis of these compounds was described previously (18, 19, 20, 21) and the structures are presented in Fig. 1 and 2. The compounds were prepared in dimethyl sulfoxide (DMSO) at 10 mg/ml and diluted 1:10 in MEM containing 2% FBS to reach a final stock concentration of 1000 μg/ml. GCV and ACV were purchased from the University of Alabama at Birmingham Hospital Pharmacy and were prepared in sterile water.
FIG. 1.
Structures of the methylenecyclopropane analogues of nucleosides.
FIG. 2.
Structures of the lipophilic phosphate prodrugs of the methylenecyclopropane analogues of nucleosides.
Antiviral assays for drug evaluation.
The efficacy of these nucleoside analogues against HCMV, MCMV, HSV-1, and HSV-2 was determined by cytopathic effect (CPE) inhibition and plaque reduction (PR) assays, as reported previously (19). VZV was tested in plaque reduction assays only. Efficacy of the analogues against EBV was determined using viral capsid antigen (VCA) expression in P3HR-1 Daudi cells by immunofluorescence and a DNA hybridization assay as reported previously (18). Inhibition of DNA synthesis was determined utilizing the Enzo Simply Sensitive Horseradish Peroxidase-AEC In Situ Detection System for EBV (Enzo Diagnostics, Farmingdale, N.Y.).
The toxicities of the compounds in HFF and MEF were determined by neutral red uptake and cell proliferation assays in HFF as described previously (18). QYL-438 and QYL-769 were further evaluated for toxicity in human lymphoblastic and bone marrow progenitor cells as described previously (26, 27). The MacSynergy II, version 1.0 (University of Michigan, 1992 release), computer program was used to calculate the 50% effective concentration (EC50), 50% cytotoxic concentration (CC50), and 50% inhibitory concentration (IC50). In general, these assays were performed only once due to a limited drug supply. However, in selected cases, two assays were performed, and in these cases the means and standard deviations are included.
RESULTS
Antiviral efficacy of the methylenecyclopropane analogues.
Thirteen methylenecyclopropane analogues and prodrugs demonstrated moderate or good activities against HCMV in both CPE inhibition and PR assays (Table 1). QYL-438 was the most potent compound and exhibited EC50 values of 0.04 and 1.2 μM in the CPE inhibition and PR assays, respectively. This was comparable to or better than GCV, which showed EC50 values of 0.39 and 2.3 μM for the corresponding assays. Good antiviral activity of the 13 compounds was also demonstrated against MCMV in plaque reduction assays, with QYL-438 (EC50 = 0.30 μM) and QYL-418 (EC50 = 0.27 μM) being the most potent compounds compared to GCV (EC50 = 2.0 μM). Several other compounds were highly efficacious as well, with EC50 values of <2.0 μM. In addition, most compounds were only moderately toxic or nontoxic in MEF as determined by visual inspection (CC50 of 100 to >500 μM) compared to GCV (CC50 >39.2 μM).
TABLE 1.
Activities of methylenecyclopropane analogues against HCMV and MCMV in HFF or MEF
| Drug | EC50 (μM) for HCMV
|
EC50 (μM) for MCMV
|
|||
|---|---|---|---|---|---|
| Determined by CPE inhibition assay | Determined by PR assay | SIa | Determined by PR assay | SI | |
| GCV | 0.39 | 2.3 | >170 | 2.0 | >19.6 |
| QYL-284A | 1.0 | 1.3 | >353 | 2.1 | >219 |
| QYL-438 | 0.04 | 1.2 | >358 | 0.3 | >1,430 |
| QYL-468 | 3.4 | 9.8 | >52.9 | 52.3 | >9.9 |
| QYL-546 | 11.2 | 15.9 | >27.1 | 0.6 | >488 |
| QYL-587A | 1.9 | 4.2 | >110 | 55 | >9.4 |
| QYL-678c | 7.6 | 25.0 | >8.2 | 3.3 | >62.4 |
| QYL-685d | 11.2 | 7.8 | 15.5 | 3.2 | 65.9 |
| QYL-769 | 2.0 | 2.4 | 136.3 | 0.37 | 595 |
| QYL-418 | 5.4 | 6.9 | 26.8 | 0.27 | 963 |
| QYL-788 | NTb | 1.9 | 94.7 | 0.55 | 538 |
| QYL-972e | 0.22 | 4.5 | 7.6 | 0.24 | 525 |
| QYL-941 | 1.5 | 5.3 | 76.2 | 0.4 | 650 |
| QYL-975f | 0.9 | 6.2 | 20.3 | 0.93 | 109 |
SI, selectivity index (CC50/EC50). EC50 values are from PR assays. CC50 values are obtained from Table 7.
NT, not tested.
Prodrug of QYL-438.
Prodrug of QYL-546.
Prodrug of QYL-941.
Prodrug of QYL-769.
The excellent antiviral activity and low toxicity of QYL-438, as well as those of QYL-769, led to their selection for further evaluation against additional laboratory and clinical HCMV strains, and both were as active as or more active than GCV (Table 2), with EC50 values ranging from 1.2 to 11.6 μM for QYL-438 and 2.2 to 10.6 μM for QYL-769, compared to GCV (EC50 of 2.3 to 9.8 μM). In addition, QYL-438 and QYL-769 demonstrated activities against all the GCV-resistant HCMV isolates tested (Table 3). QYL-769 was particularly active with a 5- to 10-fold or greater efficacy compared to GCV for most of the resistant strains tested.
TABLE 2.
Efficacies of QYL-438, QYL-769, and GCV against laboratory and clinical isolates of HCMV
| HCMV strain | EC50 (μM)a of:
|
||
|---|---|---|---|
| QYL-438 | QYL-769 | GCV | |
| AD169 (control) | 1.2 | 2.4 | 2.3 |
| Toledo (2)b | 8.6 ± 3.0 | 5.5 ± 1.3 | 9.8 ± 3.9 |
| Davis (2) | 2.5 ± 0.09 | 2.2 ± 0.62 | 5.3 ± 1.2 |
| Towne | 11.6 | 10.6 | 5.1 |
| EC (2) | 5.1 ± 4.3 | 2.2 ± 0.6 | 3.9 ± 0.8 |
| CH | 3.1 | 2.7 | 4.3 |
| Coffman (2) | 6.9 ± 6.0 | 3.1 ± 0.8 | 4.7 ± 0.6 |
| C8708/17-1-1 (3) | 9.4 ± 2.6 | 4.4 ± 3.3 | 5.9 ± 3.8 |
| C9208/3-3-1 | 6.9 | 2.2 | 4.7 |
| C9208/5-4-2 | 9.4 | 4.0 | 3.8 |
By PR assay. Some results are presented as means ± standard deviations.
Number of experiments shown in parentheses.
TABLE 3.
Efficacies of QYL-438 and QYL-769 against GCV-resistant HCMV isolates
| HCMV strain | EC50 (μM)a of:
|
||
|---|---|---|---|
| QYL-438 | QYL-769 | GCV | |
| AD169 (control) | 1.2 | 2.4 | 2.3 |
| C8704/9-1-4 (2)b | 2.7 ± 0.2 | 1.7 ± 1.3 | 32.5 ± 15.3 |
| C8805/37-1-1 (2) | 48.9 ± 5.1 | 10.3 ± 4.8 | 77.6 ± 59.9 |
| C8914-6 (2) | 10.3 ± 0.6 | 4.4 ± 2.7 | 32.9 ± 11.4 |
| C8706/13-1-1 | 12 | 7.3 | 15.3 |
| C9209/1-4-4 | 10.3 | 6.6 | 323 |
| 759RD100 | 23.2 | 16.9 | 194 |
By PR assay. Some results are presented as means ± standard deviations.
Number of experiments shown in parentheses.
The methylenecyclopropane analogues were also evaluated against HSV-1 and HSV-2, and the results indicated excellent activities of four of the drugs tested in plaque reduction and CPE inhibition assays compared to ACV (Table 4). All four compounds had similar activities against HSV-2 in both CPE inhibition and PR assays.
TABLE 4.
Activity of methylenecyclopropane analogues against HSV-1 and -2 in HFF
| Drug | EC50 (μM) for virus determined by method
|
|||
|---|---|---|---|---|
| HSV-1
|
HSV-2
|
|||
| CPE inhibition | PR | CPE inhibition | PR | |
| ACV | 3.1 | 4.3 | 6.3 | 4.3 |
| QYL-284A | 0.14 | 140 | 10.6 | 47.9 |
| QYL-438 | 61.7 | NTa | 429 | NT |
| QYL-468 | >104 | NT | >104 | NT |
| QYL-546 | 413 | NT | >430 | NT |
| QYL-587A | >460 | NT | >430 | NT |
| QYL-678 | 20.2 | 22.1 | 181 | 39.2 |
| QYL-685 | 2.7 | 5.5 | 21.5 | 12.7 |
| QYL-769 | >73.4 | NT | >73.4 | NT |
| QYL-418 | >76.4 | NT | >76.4 | NT |
| QYL-972 | 0.78 | 1.1 | 0.76 | 0.88 |
| QYL-941 | 343 | NT | 364 | NT |
| QYL-975 | 1.3 | 0.8 | 2.5 | 0.8 |
NT, not tested.
Several analogues were tested against VZV in PR assays, and most exhibited activity that was comparable to that of ACV, as shown in Table 5. The analogues were next evaluated for activity against EBV using VCA and DNA hybridization assays (Table 6). Most compounds were found to be highly active, generally similar to or better than ACV. In addition, QYL-438 and QYL-769 were further evaluated against EBV in a DNA synthesis reduction assay, and both were comparable to ACV. Toxicity results in Daudi cells indicated that most of the analogues were similar to ACV.
TABLE 5.
Activity of methylenecyclopropane analogues against VZV in HFF
| Drug | EC50 (μM) for VZV |
|---|---|
| ACV | 9.8 |
| QYL-284A | 2.5 |
| QYL-438 | 61 |
| QYL-468 | 3.6 |
| QYL-546 | 93 |
| QYL-678 | 8.1 |
| QYL-972 | 0.1 |
| QYL-941 | 23.9 |
| QYL-975 | 0.6 |
TABLE 6.
Activity of methylenecyclopropane analogues against EBV VCA expression or DNA synthesis
| Drug | EC50 (μM) for EBV:
|
CC50 (μM) in Daudi cells | SIa | |
|---|---|---|---|---|
| VCA expression | DNA synthesis | |||
| ACV | 6.3 | 1.2 | >196 | >31.1 |
| QYL-284A | 3.2 | NTb | >230 | >71.9 |
| QYL-438 | 5.6 | 1.4 | >214 | >38.2 |
| QYL-468 | <0.4 | NT | >259 | >648 |
| QYL-546 | 6.9 | NT | >215 | >31.2 |
| QYL-678 | 0.05 | NT | >103 | >2,060 |
| QYL-685 | 3.8 | NT | 26.4 | 6.9 |
| QYL-769 | 11.8 | 1.7 | 27.9 | 2.4 |
| QYL-418 | 1.2 | NT | >191 | >159 |
| QYL-972 | >98 | NT | 3.7 | <0.04 |
| QYL-941 | 192 | NT | 162 | 0.84 |
| QYL-975 | >97 | NT | 97 | <1.0 |
SI, selectivity index (CC50/EC50) for EBV VCA results.
NT, not tested.
Neutral red uptake and cell proliferation assays were performed to further evaluate the cellular cytotoxicity and inhibition of cell growth of the 13 analogues in HFF (Table 7). Several compounds were nontoxic and similar to GCV or ACV (CC50 > 392 μM), while some exhibited slight to moderate levels of toxicity. QYL-438 and QYL-769 were chosen for further evaluation in human lymphoblastic and bone marrow progenitor cells (Table 8). Both QYL-438 (IC50 = 12.9 μM) and QYL-769 (IC50 = 27.9 μM) were more toxic than GCV (IC50 > 196 μM) in cell proliferation assays in Daudi cells but were slightly less toxic than GCV in HFF. They were significantly less toxic, however, than GCV in CFU-GM and BFU-E bone marrow assays.
TABLE 7.
Toxicity of methylenecyclopropane analogues in HFF or MEF
| Drug | CC50 (μM) for neutral red uptake in:
|
IC50 (μM) for cell proliferation in HFF | |
|---|---|---|---|
| HFF | MEF | ||
| GCV | >392 | >39.2 | 157 |
| ACV | >392 | NTa | >392 |
| QYL-284A | >460 | >460 | 164 |
| QYL-438 | >429 | >429 | 278 |
| QYL-468 | >518 | >518 | 356 |
| QYL-546 | >431 | >293 | 426 |
| QYL-587A | >460 | >518 | 247 |
| QYL-678 | >206 | >206 | 206 |
| QYL-685 | 121 | 211 | 211 |
| QYL-769 | 327 | 220 | 255 |
| QYL-418 | 185 | 260 | 158 |
| QYL-788 | 180 | 296 | 67.7 |
| QYL-972 | 34.1 | 126 | 2.4 |
| QYL-941 | >404 | 260 | 243 |
| QYL-975 | 126 | 101 | 2.7 |
NT, not tested.
TABLE 8.
Inhibition of cell proliferation by QYL-438, QYL-769, and GCV in various cells
| Test type | IC50 (μM) of:
|
||
|---|---|---|---|
| QYL-438 | QYL-769 | GCV | |
| Cell proliferation, Daudi | 12.9 | 27.9 | 196 |
| Cell proliferation, HFFa | 278 | 255 | 157 |
| Bone marrow (CFU-GM) (4)b | 55.7 | 77.5 | 35.3 ± 9.7 |
| Bone marrow (BFU-E) (4) | 162 | 130 | 6.6 ± 1.0 |
Data from Table 7.
Number of experiments shown in parentheses. Results for GCV are given as means ± standard deviations.
DISCUSSION
Infection with HCMV has the potential to produce life-threatening clinical syndromes in the immunocompromised patient. Effective therapeutic agents, such as GCV, PFA, and cidofovir, have been utilized to combat the manifestations of these infections; however, the occurrence of resistant strains and adverse side effects has limited their use. Therefore, it is necessary to continue the search for novel compounds for the treatment of CMV as well as other herpesvirus infections.
The search for new nucleoside analogues for antiviral therapy has led to the synthesis of many potent compounds. Unsaturated acyclic nucleoside analogues possessing a rigid allenic residue (adenallene and cytallene) in place of a ribofuranose moiety have previously been shown to exhibit strong activity against human immunodeficiency virus (6). Thus, the replacement of a double bond in the allenic residues with a cyclopropane ring widely expanded the range of efficacy of these compounds (18, 20). This structural modification served as the motivation for the development of novel compounds with a methylenecyclopropane moiety in place of an allenic group (18, 20, 23). In addition, it has been previously reported that many lipophilic phosphate prodrugs of methylenecyclopropane analogues have demonstrated antiviral activity and have been investigated in vitro and in vivo (23, 29). As reported previously, compounds QYL-769 and QYL-438 have been evaluated against HCMV and several nonhuman CMVs, such as rat CMV, MCMV, guinea pig CMV, and rhesus monkey CMV (24). The results indicated that QYL-769 and QYL-438 had excellent activity against MCMV, rat CMV, and rhesus monkey CMV compared to GCV. Additionally, QYL-769 was very active against guinea pig CMV compared to GCV. QYL-284A, QYL-438, QYL-941, QYL-972, and QYL-769 were also evaluated in MCMV-infected mice, and all demonstrated significant activity when delivered orally, particularly QYL-769, which had activity similar to that of GCV (23).
The present study investigated 13 of the methylenecyclopropane analogues and prodrugs in vitro, and all were found to be active against HCMV and MCMV in CPE inhibition and PR assays, with activity similar to or better than that of GCV. Particularly potent was QYL-438 against HCMV, and QYL-418 and QYL-438 were particularly potent against MCMV. QYL-438 and QYL-769 were further evaluated against additional HCMV strains, and both agents were found to have activities similar to GCV against laboratory and clinical isolates and all were highly effective against GCV-resistant strains as well.
The methylenecyclopropane analogues were further tested against other herpesviruses in addition to CMV to determine their potential as therapeutic agents against these opportunistic viruses. Intravenous ACV is currently utilized for treatment of both HSV-1 encephalitis and HSV-2 genital disease and is highly effective (11). However, the lack of effective topical activity for herpes labialis or herpes genitalis indicates the need for agents effective against these diseases as well. The results of the present study indicated that some of the analogues had activity against HSV-1 and HSV-2 that was as good as that of ACV. In addition, several of the analogues were evaluated against EBV, and most were highly active, particularly QYL-438 and QYL-769, which were similar to or more active than ACV.
Cellular toxicity is a crucial factor in determination of a compound's potential as an antiviral agent in addition to efficacy. Neutral red uptake and cell proliferation assays in HFF indicated moderate or no toxicity of most of the compounds compared to GCV and ACV. In MEF, most compounds were nontoxic or moderately toxic compared to GCV. QYL-438 and QYL-769 were also evaluated for toxicity in clonogenic assays utilizing bone marrow progenitor cells. In this study, both analogues were less toxic than GCV in CFU-GM and BFU-E assays. In previous studies, the CFU-GM and BFU-E assay system has been shown to be appropriate for the determination of possible hematotoxicity and thus is considered a more sensitive assay for determining systemic or bone marrow toxicity than tissue culture cells (17, 24).
These results support the potential use of several of the methylenecyclopropane analogues and their prodrugs as antiviral agents. The tissue culture data provide convincing evidence for the excellent activities of many of these compounds, and some have been shown to have activity against MCMV in vivo (23). The efficacy and toxicity were comparable to or better than those of GCV or ACV for several of the analogues, thus suggesting the need for future evaluation of these and similar compounds.
ACKNOWLEDGMENTS
This work was supported by contracts N01-A1-35177 and N01-A1-65290 from The Antiviral Substance Program, NIAID, NIH, and research grant R01-CA32779 from the NCI, NIH.
REFERENCES
- 1.Alford C A, Britt W J. Cytomegalovirus. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 227–255. [Google Scholar]
- 2.De Castro L M, Kern E R, DeClercq E, Ghaffar A, Mayer E P, Vogt P E, Gangemi J D. Phosphonylmethoxyalkylpurine and pyrimidine derivatives for treatment of opportunistic cytomegalovirus and herpes simplex virus infections in murine AIDS. Antivir Res. 1991;16:101–114. doi: 10.1016/0166-3542(91)90062-v. [DOI] [PubMed] [Google Scholar]
- 3.Drew W L, Miner R C, Busch D F, Follansbee S E, Gullett J, Mehalko S G, Gordon S M, Owen W F, Jr, Matthews T R, Buhles W C, DeArmond B. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991;163:716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- 4.Freitas V R, Smee D F, Chernow M, Boehme R, Matthews T R. Activity of 9-(1,3-dihydroxy-2-propoxymethyl)guanine compared with that of acyclovir against human, monkey, and rodent cytomegaloviruses. Antimicrob Agents Chemother. 1985;28:240–245. doi: 10.1128/aac.28.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon A A, Gold E, Nankervis G A. Cytomegalovirus. In: Evans A S, Kaslow R A, editors. Viral infections of humans: epidemiology and control. New York, N.Y: Plenum Publishing Co.; 1997. pp. 229–251. [Google Scholar]
- 6.Hayashi S, Phadtare S, Zemlicka J, Matsukura M, Mitsuya H, Broder S. Adenallene and cytallene: acyclic nucleoside analogues that inhibit replication and cytopathic effect of human immunodeficiency virus in vitro. Proc Natl Acad Sci USA. 1988;85:6127–6131. doi: 10.1073/pnas.85.16.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern E R, Overall J C, Jr, Glasgow L A. Herpesvirus hominis infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine. J Infect Dis. 1973;128:290–299. doi: 10.1093/infdis/128.3.290. [DOI] [PubMed] [Google Scholar]
- 8.Kern E R. Animals models as assay systems for the development of antivirals. In: De Clercq E, Walker R T, editors. Antiviral drug development. New York, N.Y: Plenum Publishing Co.; 1988. pp. 149–172. [Google Scholar]
- 9.Kern E R. Preclinical evaluation of antiviral agents: in vitro and animal model testing. In: Galasso G J, Whitley R J, Merigan T C, editors. Antiviral agents and viral diseases of man. 3rd ed. New York, N.Y: Raven Press; 1990. pp. 87–123. [Google Scholar]
- 10.Kern E R. Value of animal models to evaluate agents with potential activity against human cytomegalovirus. Transplant Proc. 1991;23:152–155. [PubMed] [Google Scholar]
- 11.Kern E R. Preclinical evaluation of antiviral agents. In: Galasso G J, Whitley R J, Merigan T C, editors. Antiviral agents and human viral diseases. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 79–111. [Google Scholar]
- 12.Kern E R. Animal models for cytomegalovirus infections: murine CMV. In: Zak O, Sande M, Carbon C, Kanisky R, O'Reilly T, Kern E R, editors. Handbook of animal models of infection. New York, N.Y: Academic Press; 1999. pp. 927–934. [Google Scholar]
- 13.Mocarski E D., Jr . Cytomegalovirus biology and replication. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 173–226. [Google Scholar]
- 14.Neyts J, Balzarini J, Naesens L, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine for the treatment of murine cytomegalovirus infection in severe combined immunodeficiency mice. J Med Virol. 1992;37:67–71. doi: 10.1002/jmv.1890370112. [DOI] [PubMed] [Google Scholar]
- 15.Neyts J, Sobis H, Snoeck R, Vandeputte E, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)-cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)-guanine in the treatment of intracerebral murine cytomegalovirus infections in immuno-competent and immunodeficient mice. Eur J Clin Microbiol Infect Dis. 1993;12:269–279. doi: 10.1007/BF01967257. [DOI] [PubMed] [Google Scholar]
- 16.Overall J C, Jr, Kern E R, Glasgow L A. Effective antiviral chemotherapy in cytomegalovirus infection of mice. J Infect Dis. 1976;133(Suppl.):A237–A244. doi: 10.1093/infdis/133.supplement_2.a237. [DOI] [PubMed] [Google Scholar]
- 17.Perigaud C, Girardet J-L, Lefebvre L, Xie M-Y, Aubertin A-M, Kirn A, Gosselin G, Imbach J-L, Sommadossi J-P. Comparison of cytotoxicity of mononucleoside phosphotriester derivatives bearing biolabile phosphate protecting groups in normal human bone marrow progenitor cells. Antivir Chem Chemother. 1996;7:338–345. [Google Scholar]
- 18.Qiu Y-L, Ksebati M B, Ptak R G, Fan B Y, Breitenbach J M, Lin J-S, Cheng Y-C, Kern E R, Drach J C, Zemlicka J. (Z)- and (E)-2-((hydroxymethyl)cyclopropylidene)methyladenine and -guanine. New nucleoside analogues with a broad-spectrum antiviral activity. J Med Chem. 1998;41:10–23. doi: 10.1021/jm9705723. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y-L, Hempel A, Camerman N, Camerman A, Geiser F, Ptak R G, Breitenbach J M, Kira T, Li L, Gullen E, Cheng Y-C, Drach J C, Zemlicka J. (R)-(−) and (S)-(+)-synadenol: synthesis, absolute configuration, and enantioselectivity of antiviral effect. J Med Chem. 1998;41:5257–5264. doi: 10.1021/jm980323u. [DOI] [PubMed] [Google Scholar]
- 20.Qiu Y-L, Ptak R G, Breitenbach J M, Lin J-S, Cheng Y-C, Kern E R, Drach J C, Zemlicka J. (Z)- and (E)-2-(hydroxymethylcyclopropylidene)-methylpurines and pyrimidines as antiviral agents. Antivir Chem Chemother. 1998;9:341–352. [PubMed] [Google Scholar]
- 21.Qiu Y-L, Ptak R G, Breitenbach J M, Lin J-S, Cheng Y-C, Drach J C, Kern E R, Zemlicka J. Synthesis and antiviral activity of phosphoralaninate derivatives of methylenecyclopropane analogues of nucleosides. Antivir Res. 1999;43:37–53. doi: 10.1016/s0166-3542(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 22.Reddehase M J, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski U H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med. 1994;179:185–193. doi: 10.1084/jem.179.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rybak R J, Zemlicka J, Qui Y-L, Hartline C B, Kern E R. Effective treatment of murine cytomegalovirus infections with methylenecyclopropane analogues of nucleosides. Antivir Res. 1999;43:175–188. doi: 10.1016/s0166-3542(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 24.Sinzger C, Jahn G. Human cytomegalovirus cell tropism and pathogenesis. Intervirology. 1996;39:302–319. doi: 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 25.Smee D F, Morris J L B, Leonhardt J A, Mead J R, Holy A, Sidwell R W. Treatment of murine cytomegalovirus infections in severe combined immunodeficient mice with ganciclovir, (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine, interferon, and bropirimine. Antimicrob Agents Chemother. 1992;36:1837–1842. doi: 10.1128/aac.36.9.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommadossi J-P, Carlisle R. Toxicity of 3′-azido-3′-deoxythymidine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine for normal human hematopoietic progenitor cells in vitro. Antimicrob Agents Chemother. 1987;31:452–454. doi: 10.1128/aac.31.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommadossi J-P, Schinazi R F, Chu C K, Xie M-Y. Comparison of cytotoxicity of the (−) and (+)-enantiomer of 2′,3′-dideoxy-3′-thiacytidine in normal human bone marrow progenitor cells. Biochem Pharmacol. 1992;44:1921–1925. doi: 10.1016/0006-2952(92)90093-x. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsui Y, Kashiwai A, Kawamura N, Aiba-Masago S, Kosugi I. Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch Virol. 1995;140:1725–1736. doi: 10.1007/BF01384337. [DOI] [PubMed] [Google Scholar]
- 29.Uchida H, Kodoma E N, Yoshimura K, Maeda Y, Kosalaraksa P, Maroun V, Qiu Y-L, Zemlicka J, Mitsuya H. In vitro anti-human immunodeficiency virus activities of Z- and E-methylenecyclopropane nucleoside analogues and their phosphoro-l-alaninate diesters. Antimicrob Agents Chemother. 1999;43:1487–1490. doi: 10.1128/aac.43.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]