Figure 4.
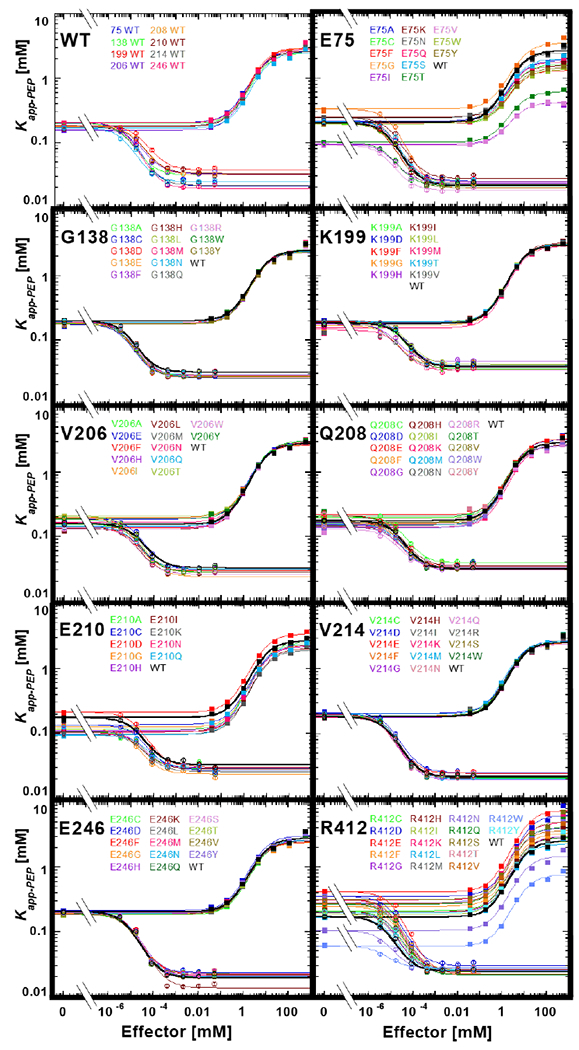
Functional characterization of hLPYK variants. The response of the apparent affinity of PEP (determined as the concentration of PEP that results in ½ maximal velocity) was measured as a function of effector concentrations for all hLPYK variants. Values for Kapp-PEP at various concentrations of both the allosteric inhibitor Ala and the allosteric activator Fru-1,6-BP were determined 5, 9, 27. In this figure, all variants made at one position are shown on the individual panels. Lines are best fits to Equation 2, and all fitted parameters for all variants are listed in Supplemental Table 3. A key for viewing how fit values are represented in this figure is included in Supplemental Figure 2. Error bars (most often smaller than the data symbols) represent errors of the fit for the Kapp-PEP values. The assays for all variants at a given position were carried out on the same day, along with a wildtype protein that is included in each panel. To represent day-to-day variability, all wildtype data sets are shown in the first panel. As described in Materials and Methods, the range of parameters from these wildtype replicates was used to establish bin size in RheoScale calculations.
