FIGURE 2.
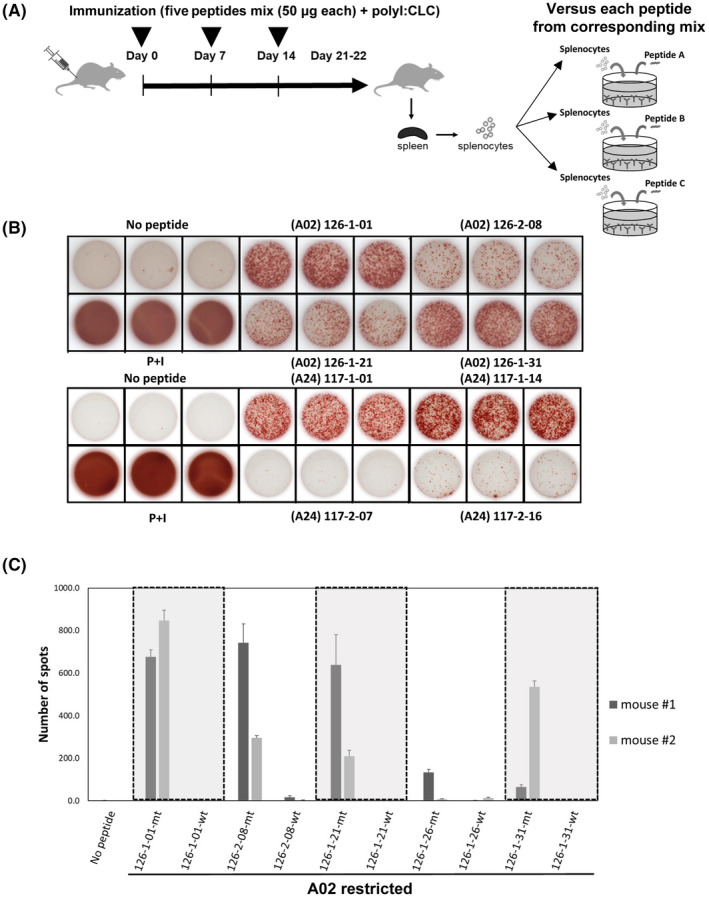
Screening of predicted neoepitopes in human leukocyte antigen (HLA) transgenic mice (Tgm). (A) Scheme of vaccinations and assays. (B) Representative pictures of ELISpot assays with A02‐restricted 126‐1‐01, 126‐2‐08, 126‐1‐21, and 126‐1‐31 peptides in vaccinated HLA‐A02 Tgm (upper pictures) and A24‐restricted 117‐1‐01, 117‐1‐14, 117‐2‐07 and 117‐2‐16 peptides in vaccinated HLA‐A24 Tgm (lower pictures). “No peptide” and “PMA + ionomycin” conditions were the negative and positive controls, respectively. (C) ELISpot assay showing mutation‐specific immune response and no immune response to corresponding wild‐type peptides in HLA‐A02 Tgm. Mt, neopeptides; wt, wild‐type peptides
