Figure 1.
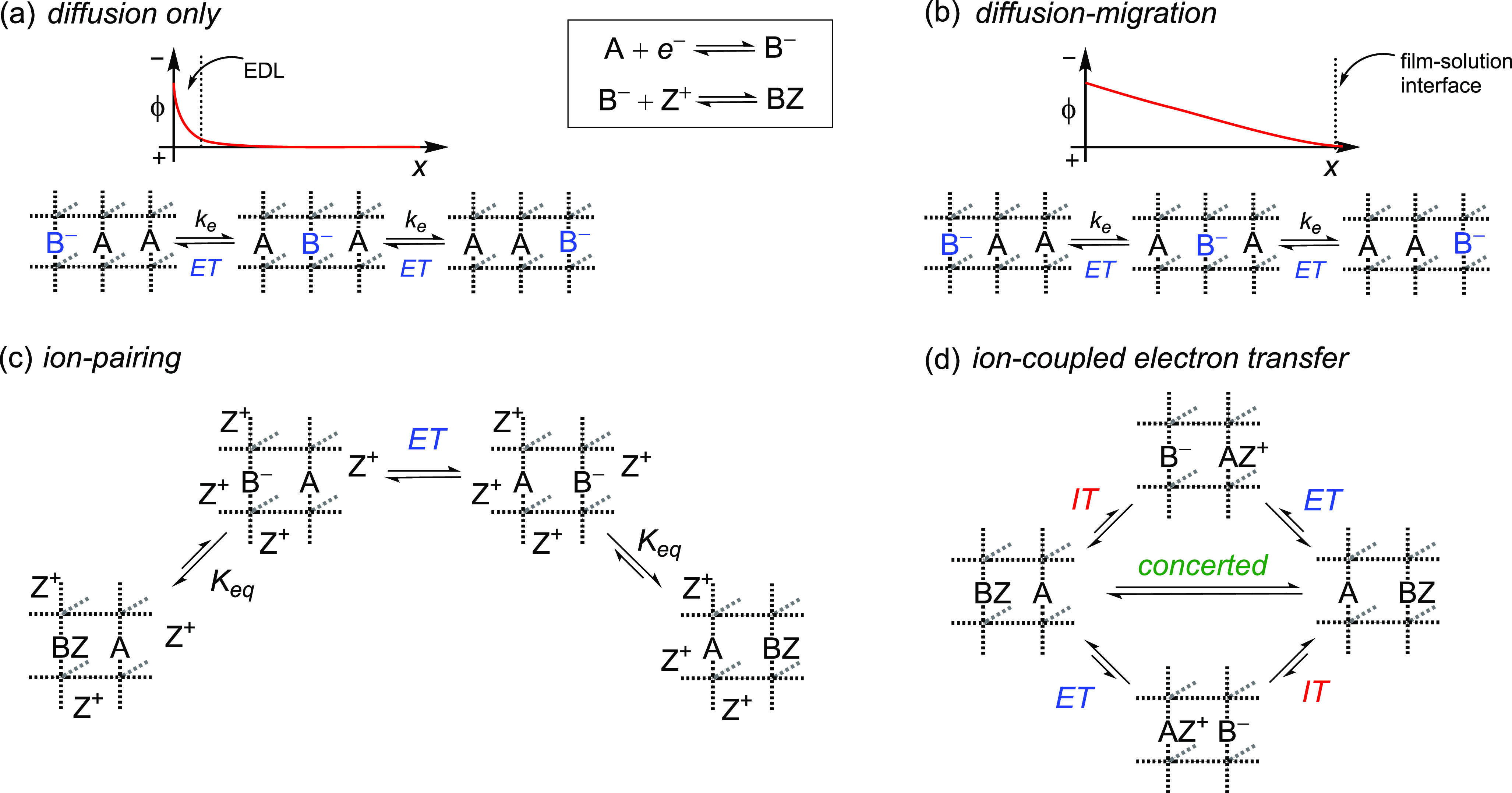
Schematic diagrams showing various microscopic mechanisms of electron-hopping through a redox-active MOF film: (a) Diffusion in the absence of any other effects, fulfilling assumptions made in eq 1; the electrostatic potential (ϕ) is dropped only over the electrical double layer (EDL) near the electrode surface and no electric field is developed within the film. (b) Charge transport by diffusion-migration, where there exists a substantial drop in electrostatic potential across the film. (c) Ion pairing electron transfer where the microscopic reactions include dissociation/association of an ion pair as well as electron self-exchange between an unpaired reduced/oxidized linker. These reactions are accompanied by migration-diffusion of redox-inactive counter ions according to the Nernst–Planck equation under an electroneutrality assumption. (d) Ion-coupled electron transfer occurring from fully associated ion-paired linkers. The microscopic self-exchange reaction follows an ion-coupled electron transfer (ICET) process, which can be represented by a square scheme showing either sequential or concerted pathways.
