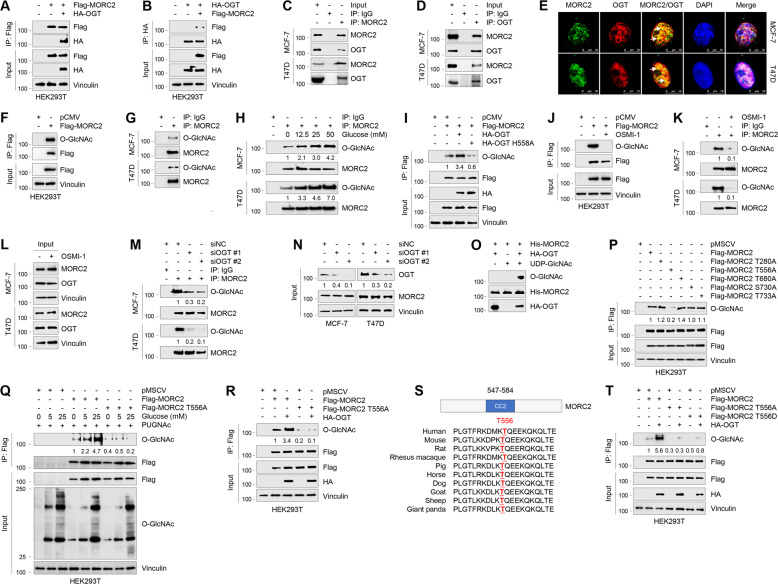
Fig. 1. OGT interacts with MORC2 and O-GlcNAcylates MORC2 at T556.
A, B HEK293T cells were transfected with Flag-MORC2 and HA-OGT alone or in combination. IP and immunoblotting analyses were performed with the indicated antibodies after 48 h of transfection. C, D Lysates from MCF-7 and T47D cells were subjected to IP and immunoblotting analysis with the indicated antibodies. E Immunofluorescence staining of MORC2 and OGT in MCF-7 and T47D cells. Cell nucleus was counterstained with DAPI. F HEK293T cells were transfected with pCMV or Flag-MORC2. IP and immunoblotting analyses were performed with the indicated antibodies after 48 h of transfection. G Lysates from MCF-7 and T47D cells were subjected to IP and immunoblotting analysis with the indicated antibodies. H MCF-7 and T47D cells were cultured in glucose- and serum-free medium for 24 h, and treated with increasing doses of glucose for 24 h. IP and immunoblotting analyses were performed with the indicated antibodies. O-GlcNAc levels were normalized to levels of immunoprecipitated MORC2. The input concerning this experiment is shown in Supplementary Fig. S1F. I HEK293T cells were transfected with Flag-MORC2, HA-OGT or HA-OGT H558A alone or in combination. After 48 h of transfection, cells were subjected to IP and immunoblotting analysis. O-GlcNAc levels were normalized to levels of immunoprecipitated Flag-MORC2. J HEK293T cells transfected with pCMV or Flag-MORC2 were treated with or without 50 μM OSMI-1 for 24 h, and then subjected to IP and immunoblotting analyses with the indicated antibodies. K, L MCF-7 and T47D cells were treated with or without 50 μM OSMI-1 for 24 h and subjected to IP and immunoblotting analyses with the indicated antibodies. In K, O-GlcNAc levels were normalized to levels of immunoprecipitated MORC2. M, N MCF-7 and T47D cells were transfected with negative control siRNA (siNC) or two independent siRNAs targeting OGT (siOGT). After 48 h of transfection, cells were subjected to IP and immunoblotting analysis with the indicated antibodies. O-GlcNAc levels were normalized to levels of immunoprecipitated MORC2 (M), and OGT levels were normalized to those of Vinculin (N). O HA-OGT was purified from HEK293T transfected with HA-OGT. Purified His-MORC2 were incubated with HA-OGT in reaction buffer in a final volume of 25 μl per sample. The samples were incubated at 37 °C for 24 h. MORC2 O-GlcNAc was detected by immunoblotting with an anti-O-GlcNAc antibody (RL2). P HEK293T cells were transfected with the indicated expression vectors. After 48 h of transfection, cells were subjected to IP and immunoblotting analysis with the indicated antibodies. O-GlcNAc levels were normalized to levels of immunoprecipitated Flag-MORC2. Q HEK293T cells were transfected with the indicated expression vectors. After 24 h of transfection, cells were cultured in glucose- and serum-free medium for 24 h and then treated with increasing doses of glucose for 24 h. IP and immunoblotting analyses were performed with the indicated antibodies. O-GlcNAc levels were normalized to levels of immunoprecipitated Flag-MORC2. R HEK293T cells were transfected with Flag-MORC2 or Flag-MORC2 T556A alone or in combination with HA-OGT. After 48 h of transfection, cells were subjected to IP and immunoblotting analysis. O-GlcNAc levels were normalized to levels of immunoprecipitated Flag-MORC2. S Alignment of OGT protein sequence among different species. T HEK293T cells were transfected with Flag-MORC2 (WT, T556A, or T556D) alone or in combination with HA-OGT. After 48 h of transfection, cells were subjected to IP and immunoblotting analysis. O-GlcNAc levels were normalized to levels of immunoprecipitated Flag-MORC2.