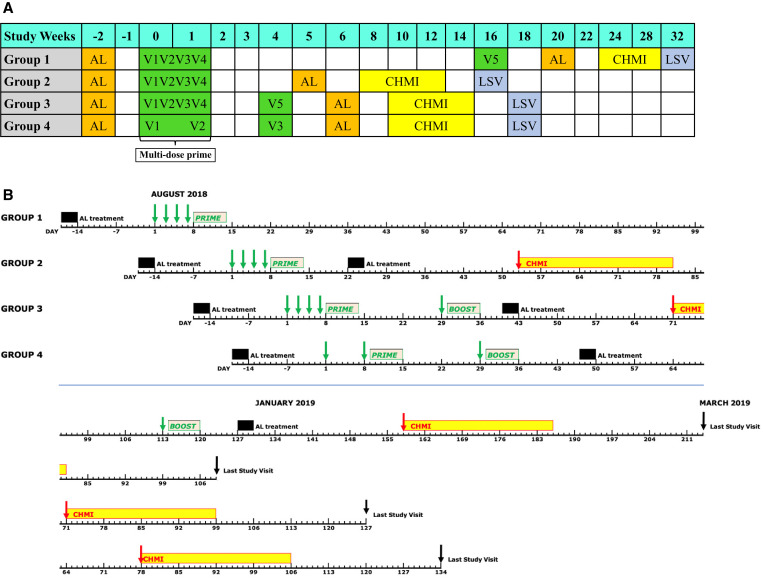
Figure 1.
Schema and execution plan for EGSPZV3 clinical trial. (A) Shows the design of the four study groups. Group 1 was the best regimen from the Warfighter 2 clinical trial, 15 Group 4 was the best regimen from the MAVACHE trial (Mordmüller, unpublished), and Groups 2 and 3 were bridging groups to explore the importance of the boost and the length of the prime-boost interval. AL = artemether/lumefantrine; V1–V5 = vaccinations 1–5; CHMI = controlled human malaria infection; LSV = last study visit. Minimum days between AL and V1: 14 days. Minimum days between AL and CHMI: 23 days. (B) Shows the execution plan for the study. Green arrows: immunizations; red arrows: CHMI; black arrows: last study visits. This figure appears in color at www.ajtmh.org.