Table 2.
Structure and bioactivity data for site B modified compounds.
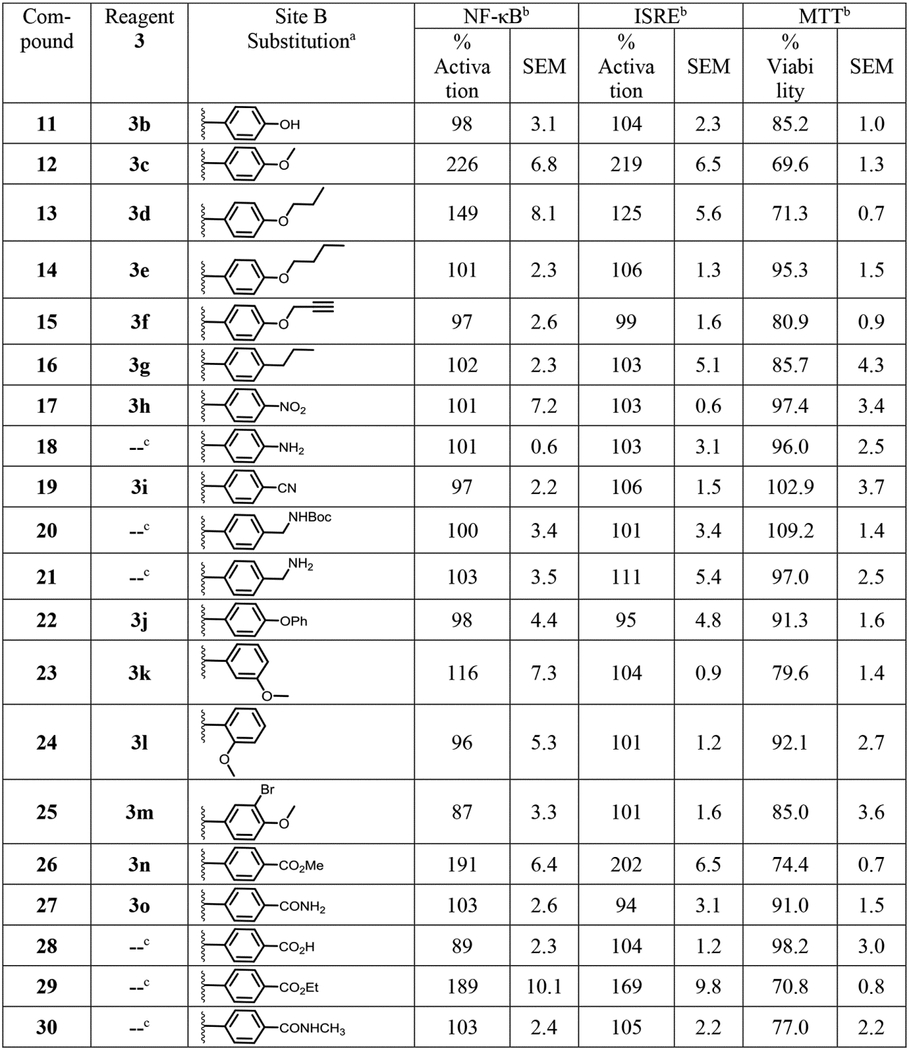
|
Compounds 11–17, 19, 22–27 were obtained by reaction of reagent 3 with 4-chloro-2,5-dimethoxyaniline (2a) as shown in Scheme 1.
The % activation values in NF-ĸB and ISRE induction assays were two point normalized between compound 1 as 200% and LPS (10 ng/mL) for NF-κB or IFN-α (100 U/mL) for ISRE as 100%. The mean SEAP response in NF-ĸB assay for compound 1 + LPS and LPS alone was 3.44 ± 0.08 and 0.56 ± 0.06 μg/mL, respectively. The mean emission ratio in ISRE assay for compound 1 + IFN-α and IFN-α alone was 1.88 ± 0.04 and 0.69 ± 0.05 μg/mL, respectively. The % viability values for compounds in MTT assay were normalized to DMSO as 100%. The mean OD value at 405 nm for DMSO was 1.24 ± 0.03. All raw values used for normalization are represented as mean ± SEM.
The compounds were synthesized as shown in Scheme 2.
