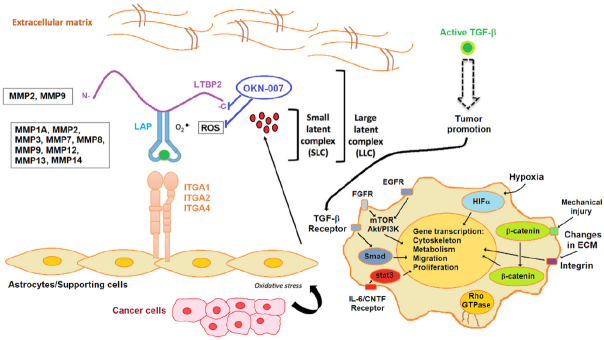
Figure 1.
Stromal activators of transforming growth factor-β (TGF-β) in the tumor microenvironment. MMP2 and MMP9 proteolytically cleave latent TGF-β binding protein (LTBP), thereby releasing latent TGF-β from the extracellular matrix. MMP1A, MMP2, MMP3, MMp7, MMP8, MMP9, MMP12, MMP13 and MMP14 activate latent TGF-β via proteolytic cleavage of the latency-associated peptide (LAP), while integrins expressed on astrocytes (ITGA1, 2 and 4) bind to the large latent complex (LLC) and activate latent TGF-β through MMP-dependent cleavage of LAP. Integrins (ITGA1, 2 and 4) bind to the LLC and induce conformational changes in the latent complex via contractile action from activated astrocytes. Reactive oxygen species (ROS) produced by activated astrocytes via the induction of oxidative stress from adjacent cancer cells can lead to the oxidation of the LAP domain and induce allosteric changes that release mature TGF-β from LAP. The mature (active) form of TGF-β can then bind to its cognate receptor and exert its tumor promoting and tumor suppressive properties. Dashed arrow indicates recruitment of the mature TGF-β protein to its cognate receptor. Other tumor-associated pathways/signaling molecules include fibroblast growth factor receptor (FGFR), EGFR, mammalian target of rapamycin (mTOR)/Akt/PI3K, HIFα (hypoxia inducible factor α), β-catenin, and stat3 (signal transducer and activator of transcription 3) (via the IL-6/CNTF receptor). Modified from Costanza et al.[104] (2017). Based on microarray and RT-PCR data from the rat F98 glioma model, comparing untreated to OKN-007-treated tumor tissue, OKN-007 is thought to act on LTBP[40], as well as ROS[105]. LTBP2, MMP1A, MMP2, MMP3, MMP7, MMP8, MMP9, MMP12, MMP13 and MMP14, were all found to be downregulated in microarray and/or RT-PCR data from the F98 glioma study[40]. Modified with permission from Dr. Towner, which was originally published in Towner et al.[40] (2019)