Abstract
The Kidney Precision Medicine Project (KPMP) seeks to establish a molecular atlas of the kidney in health and disease and improve our understanding of the molecular drivers of CKD and AKI. Herein, we describe the case of a 66-year-old woman with CKD who underwent a protocol KPMP kidney biopsy. Her clinical history included well-controlled diabetes mellitus, hypertension, and proteinuria. The patient’s histopathology was consistent with modest hypertension-related kidney injury, without overt diabetic kidney disease. Transcriptomic signatures of the glomerulus, interstitium, and tubular subsegments were obtained from laser microdissected tissue. The molecular signatures that were uncovered revealed evidence of early diabetic kidney disease adaptation and ongoing active tubular injury with enriched pathways related to mesangial cell hypertrophy, glycosaminoglycan biosynthesis, and apoptosis. Molecular evidence of diabetic kidney disease was found across the nephron. Novel molecular assays can supplement and enrich the histopathologic diagnosis obtained from a kidney biopsy.
Keywords: molecular biology, pathology, clinical nephrology
Patient Introduction
Having the privilege to be an active participant in this clinical pathologic molecular correlation (CPMC) study, I (J.B.) have observed the collaboration, inventive thinking, and dedicated adherence to evidence-based medicine by diverse experts motivated to achieve improved health outcomes (1). As you will experience in reviewing this study, the exploration of finite molecular findings to identify new correlations, that even I understand, is very exciting for us as patients to be a part of, hopefully yielding scientific advancements, motivated in part by our support and feedback with the researchers to achieve medical breakthroughs.
Clinical Case
A 66-year-old Black woman with a history of coronary artery disease, type 2 diabetes mellitus, hypertension, gastroesophageal reflux disease, hyperlipidemia, monoclonal gammopathy of unknown significance, and primary hyperparathyroidism presented to the nephrology clinic to transition care. She had a 30-year history of hypertension but no known clinical complications, including cerebrovascular accidents, transient ischemic attacks, cardiovascular infarctions, or heart failure. Her BP was well controlled on candesartan (home BPs were 120s over 70s mm Hg) with 0.5 g/g proteinuria. Previously, her BP was uncontrolled until she had a 20-pound unintentional weight loss due to lack of appetite over the 20 months prior to presentation. She was diagnosed with diabetes mellitus approximately 5 years prior to presentation. Her diabetes mellitus was well controlled, with a hemoglobin A1c below 7% on only metformin. She has no known retinopathy, neuropathy, or other complications of diabetes. Her hyperlipidemia was treated with rosuvastatin, and her reflux disease was treated with a proton pump inhibitor. She presented as a new patient after her primary care physician changed affiliations.
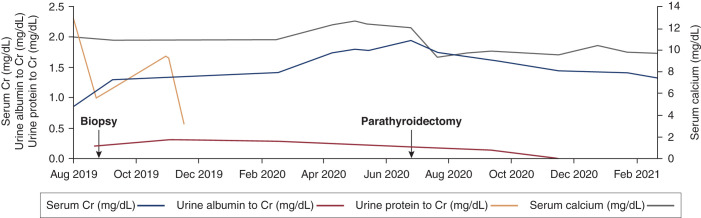
On presentation to the clinic, she had BP of 139/76 mm Hg, body mass index of 32 kg/m2, an initial serum creatinine of 0.87 mg/dl (eGFR>60), calcium of 11.1 mg/dl, and approximately 2.3 g/g of proteinuria on spot collection (Figure 1). Her rising proteinuria and diagnosis of monoclonal gammopathy of unknown significance prompted a kidney biopsy. She was offered a clinical biopsy or a protocol research biopsy through the Kidney Precision Medicine Project (KPMP) and selected the latter. Informed consent for the KPMP study was obtained.
Figure 1.
Clinical trajectory of creatinine and proteinuria. The patient’s serum creatinine (Cr; milligrams per deciliter) is plotted in relation to urine protein-Cr ratio (grams per gram), albumin-Cr ratio (grams per gram), and total calcium (milligrams per deciliter) over time.
Pathology
A 1.7-cm long core of tissue was fixed in 10% formalin; paraffin processed with sections cut at 2- to 3-µM thickness; and stained with hematoxylin and eosin (H/E), periodic acid–Schiff, trichrome, and Silver–Jones for light microscopic evaluation. Another 0.5-cm core was frozen in optimal cutting temperature (OCT) compound (Fisher, Houston, TX) at −20°C to obtain 5-µm cryostat sections for direct immunofluorescence labeling of IgG, IgM, IgA, C1q, C3, albumin, fibrinogen, and κ- and λ-light chains. A 0.2-cm portion of the core was fixed in 3% glutaraldehyde for electron microscopy evaluation.
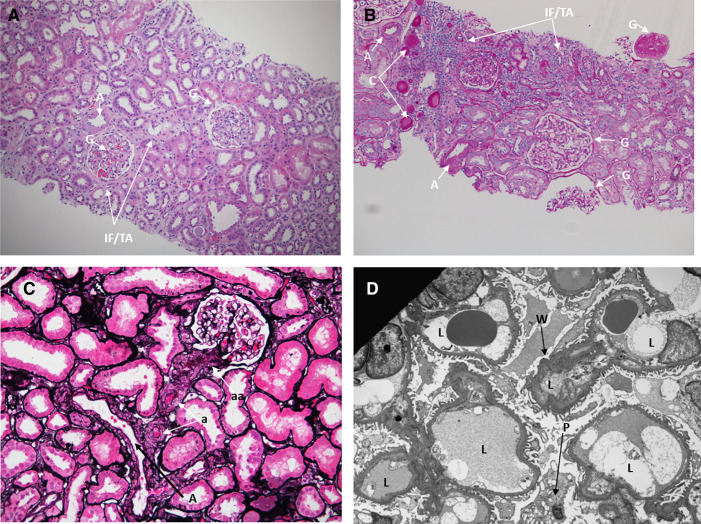
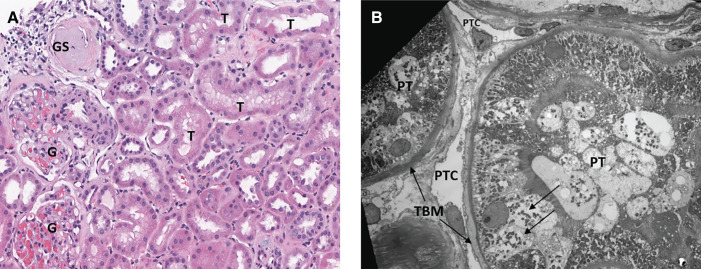
By light microscopy, the biopsy showed 80% cortex and 20% medulla. A total of 20 glomeruli, all of normal size, were identified, and two were globally sclerotic (Figure 2). Glomeruli revealed normal mesangial matrix and cellularity, with normal thickness of the glomerular capillary walls. Some cortical areas showed mild interstitial fibrosis and tubular atrophy, estimated at 10% overall, with mild chronic interstitial inflammation, occasional proteinaceous casts (Figure 2B), mild arteriosclerosis (Figure 2C), and arteriolosclerosis (Figure 2D). Glomeruli were negative for all immunofluorescence markers, including IgG, IgM, IgA, C1q, C3, albumin, fibrin, and κ- and λ-light chains. By electron microscopy, the glomerular basement membranes were of normal thickness (mean=336±28 nm) and showed mild segmental ischemic wrinkling (Figure 2D) and mild effacement of foot processes (approximately 10%). Neither electron-dense immune deposits nor matrix expansion were found. Some tubules showed mild changes of acute injury, including apical blebs, cytoplasmic vacuolar changes, and epithelial simplification (Figure 3). By electron microscopy, some tubules had mild basement membrane thickening. Peritubular capillaries had normal thickness and morphology (Figure 3B). The final pathologic diagnosis was mild hypertension-related kidney injury. There were no specific histologic findings characteristic of diabetic kidney disease (DKD) on light or electronic microscopy and immunofluorescence. Tubular basement membrane thickening is encountered in CKD of diverse etiologies, including DKD, but is specific for none (2,3). Features more specifically characteristic of DN were not present, including hyaline insudation, nodular mesangial sclerosis, or hyalinosis of the afferent and efferent arterioles.
Figure 2.
Pathology assessment of the kidney biopsy specimen. (A) A hematoxylin and eosin-stained section (×100) reveals kidney cortex with glomeruli with normal cellularity (arrows) and focal interstitial fibrosis and tubular atrophy (IF/TA; arrows). An intralobar artery (A) shows mild fibrous thickening. G, global sclerosis. (B) On a periodic acid–Schiff-stained level (×100), there are three nonsclerotic glomeruli, and none have global sclerosis (G). There is focal IF/TA and mild chronic interstitial inflammation. Two arteries (A) within normal limits and proteinaceous casts (C) are also identified. (C) Silver–Jones (×200) shows an artery (A), several arterioles (a) with mild fibrous thickening, and likely afferent arterioles (aa). (D) Electron microscopy (×2550) reveals glomerular capillary loops (L) with mild segmental ischemic wrinkling (W) of the glomerular basement membrane and a vacuolated podocyte (P).
Figure 3.
Pathologic evidence of chronic active kidney cell injury. (A) A hematoxylin and eosin-stained section (×200) shows two normal glomeruli (G) and one glomerulus with global sclerosis (GS). Tubules (T) show several mild changes of acute tubular injury, such as apical blebbing, cytoplasmic vacuoles, and epithelial simplification. Epithelial simplification refers to the loss of columnar architecture and brush borders of the proximal tubular cells and their transition to a flat, squamoid undifferentiated appearance with loss of columnar and brush border. (B) Electron microscopy (×1050) depicts proximal tubule (PT) with normal apical brush border as well as focal epithelial cytoplasmic vacuoles (arrows) and mild thickening of the basement membrane (TBM). Two peritubular capillaries (PTC) are within normal limits.
Molecular Interrogation Methods
To complement the nephropathology evaluation, the expression (i.e., the number of mRNA copies) of over 25,000 genes was measured in the glomerulus, proximal tubule, thick ascending loop of Henle, collecting duct, and interstitium, as previously described (4,5). Briefly, each segment of the nephron was identified by a rapid antibody-directed immunofluorescence reaction using specific markers for each tubular subsegment, such as megalin for the proximal tubule and uromodulin for the loop of Henle. Each labeled nephron subsegment was then laser dissected. The collected tissue for each nephron subsegment underwent bulk RNA sequencing optimized for low quantities of RNA in order to quantitate the expression of each gene.
Our patient’s gene expression was compared with the gene expression quantified from the unaffected regions of nine tumor nephrectomies or deceased donor nephrectomies without pathologic evidence of kidney disease, which were treated as normal controls. Combining RNA transcripts from all ten samples, a common overlapping set of 25,294 expressed genes was identified. The expression of genes in our patient was compared with the expression in the same nephron regions from the nine control samples in an n=1 analysis (Supplemental Material). Genes for which expression in our patient differed significantly from controls were labeled as differentially expressed genes. Groups of genes relevant to specific biologic processes (i.e., pathways) were considered enriched if we observed more differentially expressed genes in that biologic process than expected by chance.
Molecular Findings of Hypertensive Kidney Injury
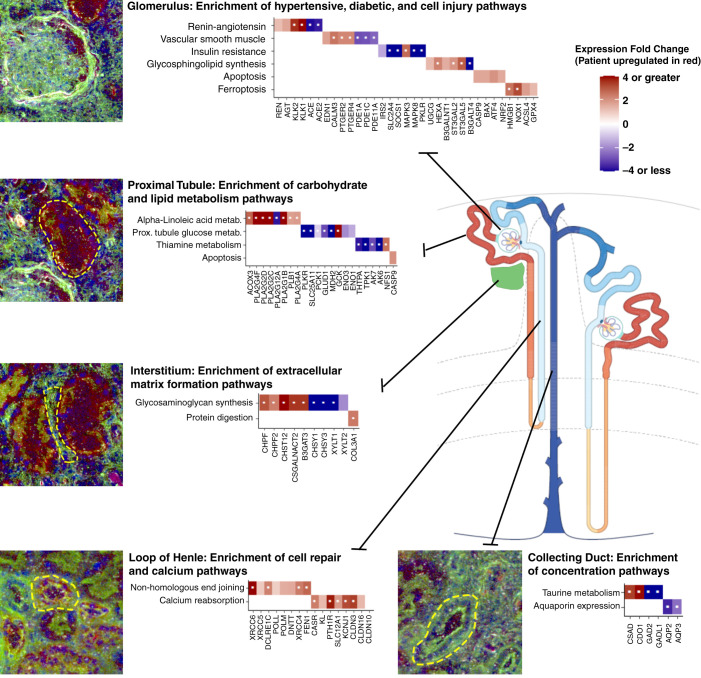
We examined the expression signatures in this patient to glean insights into the underlying biologic processes across the nephron (Figure 4). Pathways associated with hypertension-related kidney injury were upregulated in glomeruli, including the renin-angiotensin system (hsa04614) and vascular smooth muscle contraction (hsa04270). Key genes related to vasoconstriction were upregulated, including endothelin-1, calmodulin 3, and prostanoid EP2 receptor 2 and 4, consistent with previous reports of endothelial expression in hypertensive humans and rodents (6–8). In contrast, phosphodiesterase 1A (PDE1A), PDE1C, and PDE11A were downregulated. PDE enzymes contribute to vasoconstriction, and their downregulation was potentially unexpected in this hypertensive individual (9,10). Renin, an important mediator of extracellular volume retention and vasoconstriction, was upregulated; however, this may be compensatory from her candesartan treatment.
Figure 4.
Pathways and differentially expressed genes identified through regional transcriptomics. Key enriched pathways and related significant genes are depicted for each segment of the nephron and the kidney interstitium. Genes upregulated in our participant as compared with the reference nephrectomies are depicted in red. Genes downregulated are depicted in blue. Each gene depicted passed an arbitrary significance threshold of P<0.05 (except claudin-10 [CLDN10], which is depicted as a comparison). Genes labeled with a white dot passed a false discovery rate–corrected significance threshold of P<1.98 x 10-6. Immunofluorescence inset images are provided from laser microdissection microscopy. In all images, blue is DAPI (4′,6-diamidino-2-phenylindole), and green is FITC (fluorescein)-phalloidin, which labels F-actin. In the glomerulus, proximal tubule, and interstitium insets, the red channel is megalin (LDL receptor-related protein 2 [LRP2]) labeling proximal tubules. In the loop of Henle and collecting duct insets, the red channel is Tamm–Horsfall protein (uromodulin [UMOD]), which labels the loop of Henle.
Despite a dearth of histopathologic evidence of DKD, pathways associated with early changes of DKD and AKI were also enriched across the nephron.
Molecular Findings of Diabetic Kidney Disease in Glomeruli
Insulin resistance (hsa04930) was enriched in the glomerulus. Key mediators of insulin resistance, such as the insulin-regulated glucose transporter (GLUT4, SLC2A4) and insulin receptor substrate 2, were downregulated (11,12). A molecule protective from DKD progression, suppressor of cytokine signaling 1, was also downregulated. This molecule is expressed in the podocytes and mesangial cells of individuals with diabetes and mitigates the progression of DKD (13,14). The patient’s oral metformin therapy somewhat clouds the interpretation of the pathway’s direction of effect as the drug opposes the effects of chronic hyperglycemia on insulin resistance (15,16).
Additional pathways enriched in the glomerulus included the glycosphingolipid biosynthesis pathways (hsa00604 and hsa00603) and multiple glycosaminoglycan biosynthesis pathways related to chondroitin and heparin sulfate synthesis. Both glycosphingolipid synthesis (17) and glycosaminoglycan biosynthesis (18–20) mediate mesangial cell hypertrophy, matrix expansion, and loss of glomerular endothelial barrier regulation. The patient’s glucosylceramide synthase (UGCG) expression was increased. As mesangiolysis and podocytopathy may ensue, downstream injury and inflammatory pathways were also engaged, including apoptosis (upregulated caspase-9; BCL2-associated X protein; activating transcription factor 4; and NF, erythroid 2 like 2) and ferroptosis (upregulated high mobility group box 1, NADPH oxidase 1, acyl-CoA synthetase 4, and glutathione peroxidase 4) (21). Glutathione peroxidase 4 expression has also been shown to correlate with proteinuria in human FSGS (22). IFN signaling, P53 cell cycle regulation, NF-kB, IL-4, and IL-13 signaling were also enriched. Such injury signals are potentially consistent with the vacuolated podocytes and early podocyte effacement appreciated on electron microscopy. Podocyte cell loss is considered an early event in diabetic kidney injury and may precede more overt recognizable structural changes in DKD. Gene expression linked to apoptosis may provide important insight into early events in the evolution of DKD. Of note, the participant's proteinuria was out of proportion to her podocyte effacement. The proteinuric defect in this participant's glomerular filter may not be at the level of the foot process and slit diaphragm integrity but could reflect defects in the glomerular basement membrane or endothelium without extensive foot process effacement (23). Glycosphingolipid and glycosaminoglycan syntheses have been associated with the loss of endothelial barrier function.
Molecular Findings of Diabetic Kidney Disease in the Interstitium
In the kidney interstitium, glycosaminoglycan biosynthesis (hsa00532) was a highly enriched pathway with upregulation of chondroitin polymerizing factor 1 and 2, carbohydrate sulfotransferase 12, chondroitin sulfate N-acetylgalactosaminyltransferase, and β-1,3-glucuronyltransferase 3, perhaps capturing an early degree of fibrotic change in this region (19,24). Other glycosaminoglycan genes were downregulated, including chondroitin sulfate synthase 1 and 3 and xylosyltransferase 1 and 2. The upregulated expression of collagen III (COL3A1), a matrix protein of morphologically advanced DKD, was also observed. Thus, genes involved in both early and late stages of interstitial matrix accumulation were differentially expressed.
Molecular Findings of Diabetic Kidney Disease in the Proximal Tubule
In the proximal tubule, the top dysregulated pathway was α-linoleic acid metabolism, with most phospholipase A2 group genes and acyl-CoA oxidase-3 upregulated. Dietary linoleic acid supplementation has been postulated to slow DKD (25,26). Proximal tubule glucose (hsa00010) and thiamine metabolism (hsa00730) pathways also differed significantly. Key sugar metabolism intermediates, such as pyruvate kinase (PKLR), the oxoglutarate/malate carrier (SLC25A11), phosphoenolpyruvate carboxykinase 1, glutamate dehydrogenase 1, and malate dehydrogenase 2, were all differentially expressed (27,28), although it is challenging to disentangle the counter-regulatory effects of hyperglycemia and metformin utilization in a single individual. Recently, impaired proximal tubular gluconeogenesis was shown to potentiate AKI and associate with mortality in humans (29). Thiamine supplementation can restore the balance of glycolysis and glucose metabolism and is potentially beneficial in the treatment of diabetic nephropathy (30,31). Of note, thiamine metabolism was enriched in all segments of the nephron: glomerulus, proximal tubule, loop of Henle, collecting duct, and interstitium.
Molecular Findings of Injury in the Loop of Henle
As noted in the pathologic assessment, the light microscopy findings revealed proteinaceous casts and chronic low-grade kidney injury in areas of interstitial fibrosis with immune cell infiltration. On electron microscopy, changes of acute tubular injury were appreciated with apical blebbing, vacuolization, and epithelial simplification. Multiple segments of the nephron were affected by transcriptomic injury signatures. Intrinsic apoptosis was modestly enriched in the proximal tubule, with expression of caspase-9 increased in our participant. However, injury pathways were enriched in the thick ascending loop of Henle to a greater extent.
The balance of DNA repair and apoptosis is tightly regulated. Both processes are energy intensive, and the mechanisms underlying cell death or survival trajectories are of continued investigation. In contrast to the proximal tubule, DNA repair processes dominated the thick ascending loop of Henle signature. The most significant pathway was nonhomologous end joining (hsa03450), a DNA repair pathway that has been shown to be upregulated in the setting of experimental diabetic nephropathy (32). Ku70 (XRCC6) initiates the pathway, and multiple downstream pathway genes were upregulated as shown in Figure 4.
Molecular Findings Related to Primary Hyperparathyroidism
Regulated calcium reabsorption (hsa04961) was altered in the patient’s loop of Henle with upregulation of the calcium sensing receptor, Klotho, the parathyroid hormone receptor, the sodium-potassium-2-chloride transporter, renal outer medullary K+ channel (ROMK, KCNJ1), and the divalent cation transporting claudins 3 and 16 (CLDN3 and CLDN16) (33). Each of these adaptations, if present at the protein level, may conspire to impair urinary calcium excretion and unexpectedly contribute to, rather than compensate for, the patient’s remarkable hypercalcemia. Claudin-10, known to facilitate only paracellular sodium transport in the loop, was not differentially expressed.
Molecular Findings in the Collecting Duct
We conclude our march along the nephron in the collecting duct, where the most enriched pathway was taurine metabolism (hsa00430). Taurine is an osmolyte in the medullary collecting duct (34). Most taurine-related genes were downregulated with the exception of cysteine sulfinic acid decarboxylase, which has been associated with diabetes mellitus in genome-wide association studies (35). Aquaporin 2 and aquaporin 3 were also downregulated in the collecting duct of our patient as compared with the reference samples. Increases in collecting duct expression of aquaporins have been observed in murine models of DKD, which compensate for diabetic polyuria (36). Our patient had well-controlled DKD without glucosuria; thus, such compensation is unnecessary, and her aquaporin gene expression alterations may be a reflection of her serum sodium or volume status relative to those individuals undergoing a nephrectomy (the reference controls).
Clinical Follow-Up
All KPMP participants receive a full histopathologic report of their biopsy with interpretation from the KPMP team. Following the kidney biopsy, her serum creatinine rose to 1.30 mg/dl. Candesartan was titrated to manage her proteinuria. A bone marrow biopsy did not reveal a clonal plasma cell disorder. Her skeletal survey and serum free light chains were normal. Workup of her hypercalcemia was consistent with a diagnosis of primary hyperparathyroidism (parathyroid hormone level 62 pg/ml, calcium 12.1 mg/dl, ionized calcium 6.4 mg/dl, phosphate 2.0 mg/dl), and she underwent a parathyroidectomy. During her hypercalcemia workup, her serum creatinine peaked at 1.94 mg/dl before improving to 1.44 mg/dl after her parathyroidectomy.
Discussion
The classic kidney pathology interpretation of this 66-year-old woman’s kidney biopsy specimen demonstrated features common to modest hypertension-related kidney injury, mild AKI, and mild patchy interstitial fibrosis. However, the interpretation did not elucidate the underlying etiologies or provide clear evidence of evolving diabetic injury. The molecular signatures acquired in each subsegment of the nephron complemented the histopathologic interpretation and unveiled a rich pathophysiology. Evidence of molecular changes consistent with hypertensive kidney disease was identified; however, adaptations to type 2 diabetes mellitus were also readily appreciated, despite minimal evidence of DKD on histology. An understanding of the molecular pathogenesis provides an opportunity for preemptive care, when chronic irreversible injury might be avoided.
Insulin resistance in the glomerulus and deranged glucose metabolism in the proximal tubule were both observed in concert with cell injury processes. Taken together, the mild ongoing active kidney injury seen on light and electron microscopy and the molecular data support a narrative that even well-controlled hyperglycemia may potentiate subclinical kidney cell injury. The effect of diabetes mellitus is likely synergistic with those of nephrotoxic-, hemodynamic-, or hypercalcemia-related insults and may contribute to an AKI to CKD transition with progressive kidney failure. Early adaptive processes leading to matrix expansion or fibrosis were identified in the glomerular and interstitial compartments.
This study is a CPMC. Classic clinicopathologic correlations (without the molecular component) have long held educational utility, aiming to elucidate the contributors to a patient’s disease and generate hypotheses on the basis of novel observations. In this case, molecular analysis added a new dimension of understanding to the pathophysiology of kidney disease occurring in our KPMP participant, exposing important mechanisms of adaption and injury related to diabetes mellitus that were not apparent using traditional histopathology alone. A significant limitation of this study is the use of tumor and deceased donor nephrectomies as reference controls, which are often not normal. Although no signs of DKD were present in these samples, clinical data were not present to exclude clinical diabetes mellitus in all cases.
KPMP aims to redefine common CKDs and acute kidney diseases by using molecular phenotyping to enhance our clinical and histopathologic diagnoses (37). Given the paucity of advances in kidney disease therapy, the hope is that these new definitions will ultimately improve relevant clinical outcomes. The laser microdissection transcriptomics used in this study is one of many interrogation technologies that contribute to a molecular atlas of the kidney (38) hosted at https://www.kpmp.org/. Going forward, this atlas may help nephrologists and nephropathologists establish individualized diagnoses and, perhaps, prove transformative to the health of patients with CKD.
Patient Conclusion
The patient members of the KPMP consortium are thrilled by this study as an example of how a molecular atlas might advance the early detection, prevention, and specific care for individuals with chronic kidney conditions. Specifically, this abstract is the vanguard of the patient-driven clinical care advancements from KPMP, fostering the collaboration between physicians, scientists, and patients and resulting in improved health outcomes and quality of life (39). This CPMC patient study, through physician-patient knowledge and transparency, illustrates how integration of novel molecular methodology with clinical information may ultimately help practitioners diagnose and treat kidney disease and help patients with disease prevention, understanding, and motivation to self-accountability of hypertension and comorbidity management.
Disclosures
C.E. Alpers reports consultancy agreements with AstraZeneca, Mantra Bio, and Travere; research funding from Boehringer-Ingelheim and Sana; and serving as a member of the editorial boards of American Journal of Kidney Diseases, American Journal of Pathology, CJASN, Journal of Nephrology, Kidney360, and Laboratory Investigation. J. Bebiak reports employment with Greenspire Solutions and honoraria from the National Institutes of Health KPMP via Indiana University. E.D. Poggio reports employment with Cleveland Clinic; consultancy agreements with Renalytix; and honoraria from CareDx, Novartis, and Reata. R.D. Toto reports consultancy agreements with ACI, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Medscape, Otsuka, Reata Pharmaceuticals, Relypsa, and Vifor Pharma; honoraria from ACI, Akebia, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Medscape, Otsuka, Reata Pharmaceuticals, Relypsa, and Vifor; and scientific advisor or membership with ACI, Akebia, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Medscape, Otsuka, Relypsa, Reata Pharmaceuticals, and Vifor. J.R. Torrealba reports honoraria for speaking engagements with Abbvie, Pfizer, and Roche/Ventana. All remaining authors have nothing to disclose.
Funding
KPMP (https://www.kpmp.org/) is funded by National Institute of Diabetes and Digestive and Kidney Diseases grants U2CDK114886, UH3DK114861, UH3DK114866, UH3DK114870, UH3DK114908, UH3DK114915, UH3DK114926, UH3DK114907, UH3DK114920, UH3DK114923, UH3DK114933, and UH3DK114937. This work was also supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K08DK107864 (to M.T. Eadon).
Supplementary Material
Acknowledgments
We acknowledge Dr. Michael T. Valerius for his illustrative nephron schematic and the Indiana University Center for Medical Genomics Core.
All authors conceptualized the study, were responsible for analysis and interpretation of data, and were responsible for drafting/revising and final approval of the version to be published.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10350721/-/DCSupplemental.
References
- 1.Tuttle KR, Knight R, Appelbaum PS, Arora T, Bansal S, Bebiak J, Brown K, Campbell C, Cooperman L, Corona-Villalobos CP, Dighe A, de Boer IH, Hall DE, Jefferson N, Jolly S, Kermani A, Lee SC, Mehl K, Murugan R, Roberts GV, Rosas SE, Himmelfarb J, Miller RT; Kidney Precision Medicine Project : Integrating patient priorities with science by community engagement in the Kidney Precision Medicine Project. Clin J Am Soc Nephrol 16: 660–668, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziyadeh FN: Renal tubular basement membrane and collagen type IV in diabetes mellitus. Kidney Int 43: 114–120, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Brito PL, Fioretto P, Drummond K, Kim Y, Steffes MW, Basgen JM, Sisson-Ross S, Mauer M: Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int 53: 754–761, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Barwinska D, El-Achkar TM, Melo Ferreira R, Syed F, Cheng YH, Winfree S, Ferkowicz MJ, Hato T, Collins KS, Dunn KW, Kelly KJ, Sutton TA, Rovin BH, Parikh SV, Phillips CL, Dagher PC, Eadon MT; Kidney Precision Medicine Project : Molecular characterization of the human kidney interstitium in health and disease. Sci Adv 7: eabd3359, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barwinska D, Ferkowicz MJ, Cheng YH, Winfree S, Dunn KW, Kelly KJ, Sutton TA, Rovin BH, Parikh SV, Phillips CL, Dagher PC, El-Achkar TM, Eadon MT; Kidney Precision Medicine Project : Application of laser microdissection to uncover regional transcriptomics in human kidney tissue. J Vis Exp 60: 10.3791/61371, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffrin EL, Deng LY, Sventek P, Day R: Enhanced expression of endothelin-1 gene in resistance arteries in severe human essential hypertension. J Hypertens 15: 57–63, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Schiffrin EL, Larivière R, Li JS, Sventek P: Enhanced expression of the endothelin-1 gene in blood vessels of DOCA-salt hypertensive rats: Correlation with vascular structure. J Vasc Res 33: 235–248, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Patel JA, Shen L, Hall SM, Benyahia C, Norel X, McAnulty RJ, Moledina S, Silverstein AM, Whittle BJ, Clapp LH: Prostanoid EP2 receptors are up-regulated in human pulmonary arterial hypertension: A key anti-proliferative target for treprostinil in smooth muscle cells. Int J Mol Sci 19: E2372, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mergia E, Stegbauer J: Role of phosphodiesterase 5 and cyclic GMP in hypertension. Curr Hypertens Rep 18: 39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F: Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: Target for reverse-remodeling therapy. Circulation 115: 2331–2339, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Liu G, Guo J, Su Z: The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14: 1483–1496, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G: Selective insulin resistance in the kidney. BioMed Res Int 2016: 5825170, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, Sanz A, Blanco J, Mezzano S, Ortiz A, Egido J, Gomez-Guerrero C: Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol 21: 763–772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recio C, Lazaro I, Oguiza A, Lopez-Sanz L, Bernal S, Blanco J, Egido J, Gomez-Guerrero C: Suppressor of cytokine signaling-1 peptidomimetic limits progression of diabetic nephropathy. J Am Soc Nephrol 28: 575–585, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira GD, Germeyer A, de Barros Machado A, do Nascimento TL, Strowitzki T, Brum IS, von Eye Corleta H, Capp E: Metformin modulates PI3K and GLUT4 expression and Akt/PKB phosphorylation in human endometrial stromal cells after stimulation with androgen and insulin. Eur J Obstet Gynecol Reprod Biol 175: 157–162, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Zhou Y, Liu Y, Ping J, Shou Q, Chen F, Ruo R: Metformin improves hepatic IRS2/PI3K/Akt signaling in insulin-resistant rats of NASH and cirrhosis. J Endocrinol 229: 133–144, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Subathra M, Korrapati M, Howell LA, Arthur JM, Shayman JA, Schnellmann RG, Siskind LJ: Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol 309: F204–F215, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster RR, Armstrong L, Baker S, Wong DW, Wylie EC, Ramnath R, Jenkins R, Singh A, Steadman R, Welsh GI, Mathieson PW, Satchell SC: Glycosaminoglycan regulation by VEGFA and VEGFC of the glomerular microvascular endothelial cell glycocalyx in vitro. Am J Pathol 183: 604–616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lensen JF, van der Vlag J, Versteeg EM, Wetzels JF, van den Heuvel LP, Berden JH, van Kuppevelt TH, Rops AL: Differential expression of specific dermatan sulfate domains in renal pathology. PLoS One 10: e0134946, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolset SO, Reinholt FP, Jenssen T: Diabetic nephropathy and extracellular matrix. J Histochem Cytochem 60: 976–986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Zhao Y, Yang HZ, Wang YJ, Chen Y: HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep 41: BSR20202924, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidhom EH, Kim C, Kost-Alimova M, Ting MT, Keller K, Avila-Pacheco J, Watts AJ, Vernon KA, Marshall JL, Reyes-Bricio E, Racette M, Wieder N, Kleiner G, Grinkevich EJ, Chen F, Weins A, Clish CB, Shaw JL, Quinzii CM, Greka A: Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease. J Clin Invest 131: 141380, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farquhar MG: The glomerular basement membrane: Not gone, just forgotten. J Clin Invest 116: 2090–2093, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dos Santos ALT, Duarte CK, Santos M, Zoldan M, Almeida JC, Gross JL, Azevedo MJ, Lichtenstein AH, Zelmanovitz T: Low linolenic and linoleic acid consumption are associated with chronic kidney disease in patients with type 2 diabetes. PLoS One 13: e0195249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barcelli UO, Weiss M, Beach D, Motz A, Thompson B: High linoleic acid diets ameliorate diabetic nephropathy in rats. Am J Kidney Dis 16: 244–251, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Lash LH: Mitochondrial glutathione in diabetic nephropathy. J Clin Med 4: 1428–1447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulder S, Hammarstedt A, Nagaraj SB, Nair V, Ju W, Hedberg J, Greasley PJ, Eriksson JW, Oscarsson J, Heerspink HJL: A metabolomics-based molecular pathway analysis of how the sodium-glucose co-transporter-2 inhibitor dapagliflozin may slow kidney function decline in patients with diabetes. Diabetes Obes Metab 22: 1157–1166, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legouis D, Ricksten SE, Faivre A, Verissimo T, Gariani K, Verney C, Galichon P, Berchtold L, Feraille E, Fernandez M, Placier S, Koppitch K, Hertig A, Martin PY, Naesens M, Pugin J, McMahon AP, Cippà PE, de Seigneux S: Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat Metab 2: 732–743, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Karachalias N, Babaei-Jadidi R, Rabbani N, Thornalley PJ: Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia 53: 1506–1516, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Pácal L, Tomandl J, Svojanovsky J, Krusová D, Stepánková S, Rehorová J, Olsovsky J, Belobrádková J, Tanhäuserová V, Tomandlová M, Muzík J, Kanková K: Role of thiamine status and genetic variability in transketolase and other pentose phosphate cycle enzymes in the progression of diabetic nephropathy. Nephrol Dial Transplant 26: 1229–1236, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Tunçdemir M, Öztürk M: Regulation of the Ku70 and apoptosis-related proteins in experimental diabetic nephropathy. Metabolism 65: 1466–1477, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M, Günzel D: Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci U S A 114: E219–E227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones DP, Miller LA, Chesney RW: The relative roles of external taurine concentration and medium osmolality in the regulation of taurine transport in LLC-PK1 and MDCK cells. Pediatr Res 37: 227–232, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Kawabata Y, Nishida N, Awata T, Kawasaki E, Imagawa A, Shimada A, Osawa H, Tanaka S, Takahashi K, Nagata M, Yasuda H, Uchigata Y, Kajio H, Makino H, Yasuda K, Kobayashi T, Hanafusa T, Tokunaga K, Ikegami H: Genome-wide association study confirming a strong effect of hla and identifying variants in csad/lnc-itgb7-1 on chromosome 12q13.13 associated with susceptibility to fulminant type 1 diabetes. Diabetes 68: 665–675, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Satake M, Ikarashi N, Kagami M, Ogiue N, Toda T, Kobayashi Y, Ochiai W, Sugiyama K: Increases in the expression levels of aquaporin-2 and aquaporin-3 in the renal collecting tubules alleviate dehydration associated with polyuria in diabetes mellitus. Biol Pharm Bull 33: 1965–1970, 2010 [DOI] [PubMed] [Google Scholar]
- 37.de Boer IH, Alpers CE, Azeloglu EU, Balis UGJ, Barasch JM, Barisoni L, Blank KN, Bomback AS, Brown K, Dagher PC, Dighe AL, Eadon MT, El-Achkar TM, Gaut JP, Hacohen N, He Y, Hodgin JB, Jain S, Kellum JA, Kiryluk K, Knight R, Laszik ZG, Lienczewski C, Mariani LH, McClelland RL, Menez S, Moledina DG, Mooney SD, O’Toole JF, Palevsky PM, Parikh CR, Poggio ED, Rosas SE, Rosengart MR, Sarwal MM, Schaub JA, Sedor JR, Sharma K, Steck B, Toto RD, Troyanskaya OG, Tuttle KR, Vazquez MA, Waikar SS, Williams K, Wilson FP, Zhang K, Iyengar R, Kretzler M, Himmelfarb J; Kidney Precision Medicine Project : Rationale and design of the Kidney Precision Medicine Project. Kidney Int 99: 498–510, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Achkar TM, Eadon MT, Menon R, Lake BB, Sigdel TK, Alexandrov T, Parikh S, Zhang G, Dobi D, Dunn KW, Otto EA, Anderton CR, Carson JM, Luo J, Park C, Hamidi H, Zhou J, Hoover P, Schroeder A, Joanes M, Azeloglu EU, Sealfon R, Winfree S, Steck B, He Y, D’Agati V, Iyengar R, Troyanskaya OG, Barisoni L, Gaut J, Zhang K, Laszik Z, Rovin BH, Dagher PC, Sharma K, Sarwal MM, Hodgin JB, Alpers CE, Kretzler M, Jain S: A multimodal and integrated approach to interrogate human kidney biopsies with rigor and reproducibility: Guidelines from the Kidney Precision Medicine Project. Physiol Genomics 53: 1–11, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuttle KR, Bebiak J, Brown K, Campbell C, Dighe A, Hyashi L, Jefferson N, Roberts GV, Stutzke C, Knight R; Kidney Precision Medicine Project : Patient perspectives and involvement in precision medicine research. Kidney Int 99: 511–514, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.