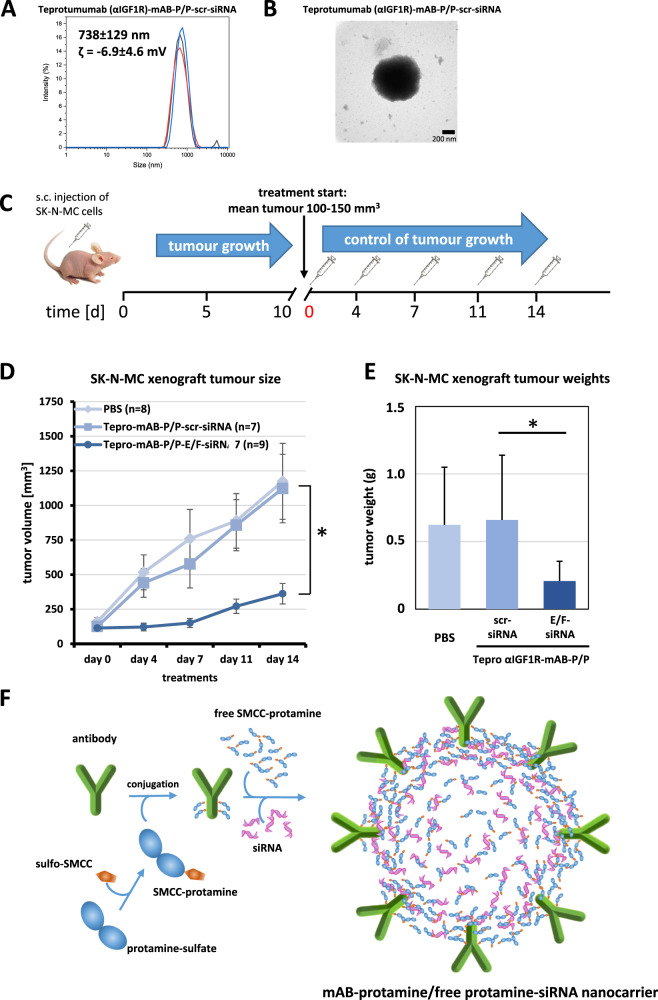
Fig. 8. Ewing sarcoma xenograft tumour growth is inhibited upon knockdown of oncogenic EWS-FLI1 translocation product through systemic therapy with αIGF1R-mAB-protamine-siRNA-protamine nanocarriers.
A Dynamic light scattering spectroscopy (DLS) of teprotumumab (αIGF1R)-mAB-protamine-siRNA-protamine nanocarriers reveals a particles size of 738 ± 129 nm with a zeta-potential of −6.9 ± 4.6 mV. B αIGF1R-mAB-P/P-scrm siRNA nanoparticles were left to form for 2 h and subjected to electron microscopy on copper grids by phospho-Wolfram negative staining. C Treatment scheme of the in vivo experiments. Nanoparticles were given intraperitoneally as visualised. D, E Results of systemic in vivo application of targeted nanocarriers on SK-N-MC xenograft tumours. D Tumour growth curves SK-N-MC treated with αIGF1R-mAB teprotumumab (“Tepro”)-protamine-siRNA/P nanoparticles (means ± SEM; two-sided t-test, *p < 0.05). E Weight statistics of the excised tumours at the end of the experiment (mean ± SD. Two-sided t-test, *p < 0.05). F Illustration of a cross section through an idealised nanoparticle structure fulfilling the conditions for an effective antibody-protamine-siRNA-SMCC-protamine nanocarrier complex deduced from our experiments. Electrostatic binding bridges are formed between mAB, with some protamines (cationic) coupled to the targeting antibody, siRNA (anionic), and free SMCC-protamine (cationic). The nanostructures assemble spontaneously into the optimal and most stable electrostatic status and function as nanocarriers for siRNA.