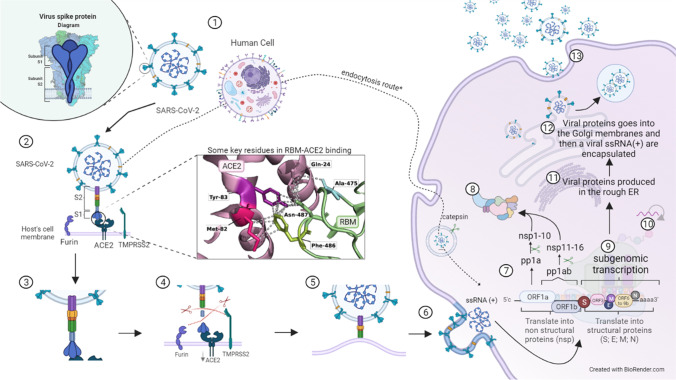
Fig. 2.
Role of spike protein in the SARS-CoV-2 infection mechanism. 1 The S protein is used by SARS-CoV-2 to interact with the host cell receptor. The host cell has several different receptors and polysaccharides in its membranes. In this step-by-step figure of the cycle, we focus on the major receptor used, which is the ACE2 receptor, but SARS-CoV-2 may also interact with other cell receptors. 2 Protein S attacks its target cells: the RBM (in RBD) interacts with the ACE2 receptor. In this process, A475 and F486 in the RBM seem to be the key residues to connect. In this step, it is important to note 2 additional receptors important for viral entry: furin and TMPRSS2. 3 Interaction between HR1 and HR2: the connection causes conformational changes in S2, where HR1 and HR2 motifs interact to start the formation of the six-helix bundle. 4 Proteolytic activation of spike: after S-ACE2 binding, proteolytic activation of spike occurs when the additional receptors cleave the protein, exposing the fusion peptide (FP). While furin cleaves the polybasic cleavage site (PRRAR) between S1 and S2, TMPRSS2 cleaves an S2 site. 5 Approximation between viral and host membranes: the FP approximates the viral and host membranes together with the six-helix bundle, leading to fusion and viral entry. 6 Fusion and viral entry: fusion of the membranes releases the viral RNA into the cytoplasm of the host cell. 7 Translation of replicase genes in the viral RNA genome: since the RNA of SARS-CoV-2 is a + ssRNA, it can be translated directly into polyproteins: ORFa will be translated in pp1a, which will be further cleaved in nspn 1 to 10, and by a − 1 ribosomal frame shifting, ORF1a and ORF1b will translate to pp1b, which will generate nsps 11 to 16. 8 Replicase-transcriptase complex: the viral RNA-dependent RNA polymerase (RdRp), composed of nsp12, nsp7, and 8 along with other nsps, condenses and assembles in the rough endoplasmic reticulum (ER) membrane-bound ribonucleoprotein complex, the so-called replicase-transcriptase complex (RTC) that directs replication, transcription, and maturation of the viral genome and subgenomic mRNAs. 9 Subgenomic transcription: the direct translation of the viral genome produces several ( −) subgenomic RNAs (sgRNAs) from the structural protein genes and ORF3 and ORF6 to 9, which are synthesized in a full-length negative strand RNA combining varying lengths of the 3′ end of the genome with the 5′ leader sequence. 10 Replication: the full-length ( −) RNA is also used as a mold to replicate the ( +) ssRNA of the virus that will form the new virion. 11 Structural protein synthesis: the ( −) sgRNA is used as a mold to synthesize subgenomic ( +) mRNAs, which are then translated into M, S, E, and N proteins and processed as needed. 12 Encapsulation: after translation, the structural proteins are inserted into the endoplasmic reticulum and continue to their intermediate compartment, where the replicated ( +) ssRNA will interact with N protein, forming the nucleocapsid that will be enveloped in the ER-Golgi intermediate. 13 Transport and exocytosis: the virions are transported by vesicles and released by exocytosis