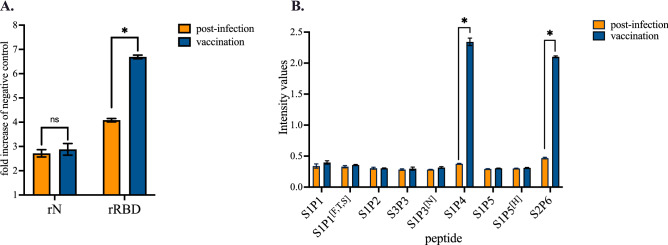
Figure 4.
Antigenic reactivity of synthetic peptides with immune sera from a COVID-19 immune subject who received comirnaty vaccine. Serum samples from a COVID-19 patient were collected few weeks after recovery of SARS-CoV-2 infection (post-infection) and then two weeks after the injection of a single dose of comirnaty vaccine (vaccination). Vaccine administration was performed three months after COVID-19 recovery. In (A), serum samples at dilution 1:100 were assayed for the detection of antibodies against SARS-CoV-2 N and spike proteins by indirect ELISA using recombinant rN and rRBD proteins for antigen-based antibody capture. A pool of serum samples from infection-naïve individuals (Table S3) served as negative control serum. The intensity values of serum samples were measured at O.D. 450 nm and their immune reactivity was estimated as a fold increase of intensity values obtained with negative control serum. The results are the mean (± SEM) of three replicates. Statistically significant comparisons are shown as * p < 10–4 (ns: non-statistically significant, p > 0.05). In (B), serum samples at dilution 1:50 were assayed for the detection of specific antibodies through peptide-based ELISA. The intensity values of serum samples were measured at O.D. 450 nm. The results are the mean (± SEM) of three replicates. Paired t tests on experimental points between post-infection and vaccination immune were performed and statistically significant comparisons are shown as * p < 10–4. Differences between experimental points considered as non-statistically significant are not shown.