Figure 2 ∣. p23tail-helix interactions and effect on GR ligand binding.
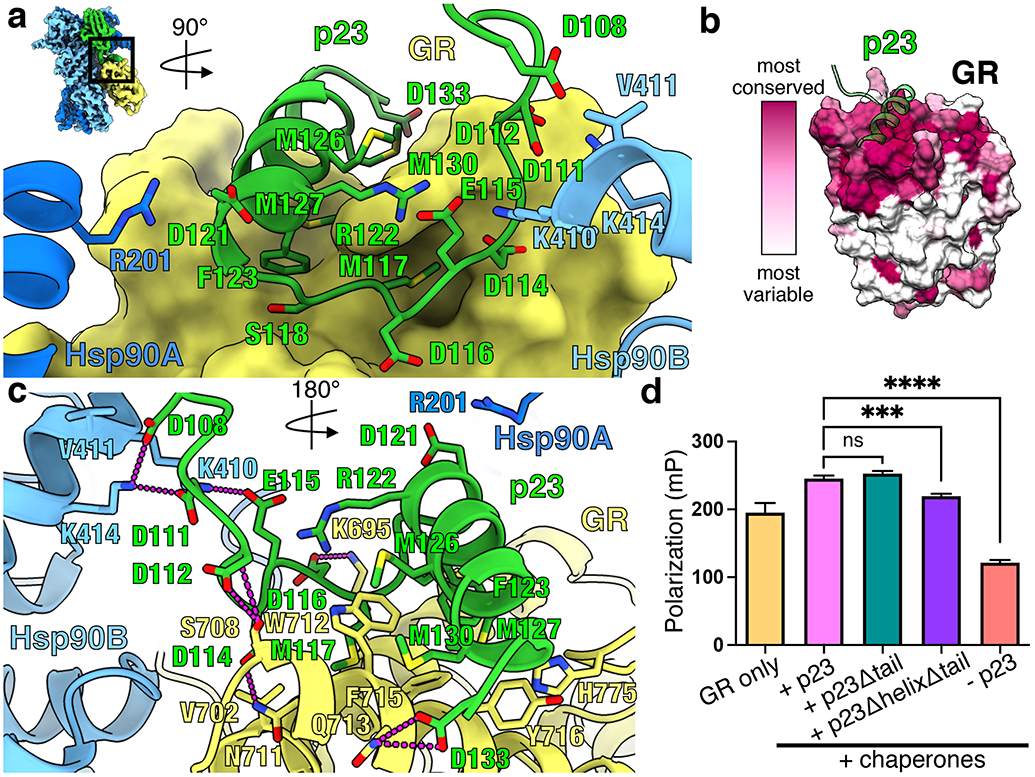
a, Interface between the p23tail-helix (green), GR (yellow, surface representation), Hsp90A (dark blue), and Hsp90B (light blue). The p23tail-helix (p23119-131) binds GR, while the preceding p23 loop (p23108-118) interacts with GR and Hsp90. b, GR protein sequence conservation mapped onto the GR surface colored from most variable (white) to most conserved residues (maroon). The p23tail-helix (green) is overlaid to indicate the p23:GR interface. c, Interface between the p23tail-helix, GR, and Hsp90 showing interacting side chains and hydrogen bonds (dashed pink lines). d, Equilibrium binding of 20nM fluorescent dexamethasone to 250nM GR with chaperones and p23 tail mutants measured by fluorescence polarization (mean±SD). n=3 biologically independent samples per condition (n=6 biologically independent samples for the GR only condition). See Extended Data Fig. 7b for data points. Significance was evaluated using a one-way ANOVA (F(3,8) = 636.2; p < 0.0001) with post-hoc Dunnett’s test (n.s. P ≥ 0.05; * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001). P-values: p(p23 vs. p23Δtail) = 0.1512, p(p23 vs. p23ΔhelixΔtail) = 0.0002, p(p23 vs. no p23) = <0.0001.
