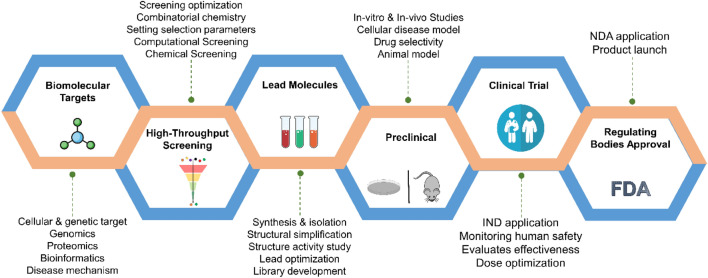
Fig. 1.
Overview of the drug discovery and development process. The process includes various stages such as (1) target identification, in which biologically relevant targets for a particular disease condition is selected for drug development, (2) high-throughput screening of compound library to identify hit compounds, (3) lead identification and lead optimization, in which the identified hit molecules are optimizing for their potency, selectivity and ADMET properties, (4) preclinical studies, in which the optimized molecules are tested in animal models to study their pharmacokinetic properties and therapeutic potential, and finally, (5) clinical trials, where the drug candidates are tested on human subjects in four phases to establish their safety and efficacy followed by regulatory approval, molecules which showed good pharmacokinetic properties, potency, therapeutic efficacy and least side effects are approved by regulatory agency for marketing and a drug become available in the clinic