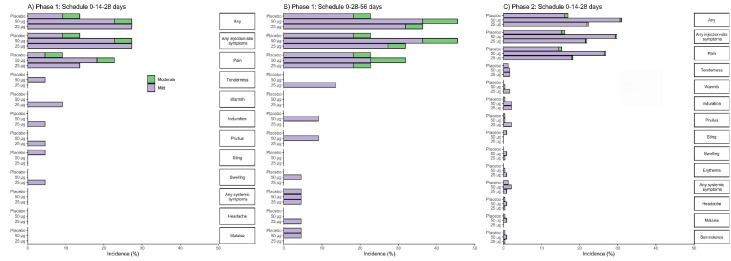
Figure 2.
Percentage of participants in each phase of the trial according to the occurrence of adverse reactions, by group and vaccination schedule.
The percentage of participants in each study group (RBD 25 µg, RBD 50 µg, Placebo) with adverse reactions according to the maximum FDA (Food and Drug Administration) toxicity grade (mild or moderate) from first dose up to 14 days after third dose is plotted by signs or symptoms. Participants who reported 0 events make up the remainder of the 100%. Panel A: phase 1 (schedule 0-14-28 days); Panel B: phase 1 (schedule 0-28-56 days); Panel C: phase 2 (schedule 0-14-28 days).
The percentage of participants in each study group (RBD 25 µg, RBD 50 µg, Placebo) with adverse reactions according to the maximum FDA (Food and Drug Administration) toxicity grade (mild or moderate) from first dose up to 14 days after third dose is plotted by signs or symptoms. Participants who reported 0 events make up the remainder of the 100%. Panel A: phase 1 (schedule 0-14-28 days); Panel B: phase 1 (schedule 0-28-56 days); Panel C: phase 2 (schedule 0-14-28 days).