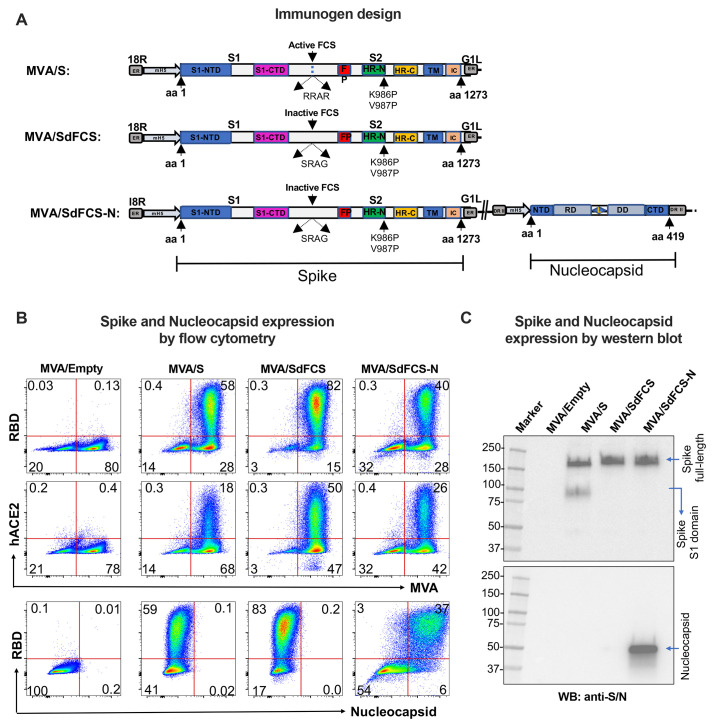
Fig. 1. Schematics designs and characterization of recombinant Modified Vaccinia Ankara (MVA)-based SARS-CoV-2 vaccines expression.
(A) Domains that represented in MVA/S, MVA/SdFCS, and MVA/SdFCS-N constructs are colored. Recombinant spike (S) and Nucleocapsid (N) inserts were cloned in the essential region (ER) in between I8R and G1L, and deletion region II (DR II) respectively, under modified H5 (mH5) promoter. NTD – N terminal domain; CTD – C terminal domain; FP – Fusion peptide; HR-N – Heptad repeat N; HR-C – Heptad repeat C; TM – Transmembrane anchor; IC – intracellular tail; Active FCS (FCS – Furin cleavage site – RRAR); Inactive FCS (FCS mutation – SRAG); RD – RNA binding domain; L – Linker; and DD – Dimerization domain. Arrows represents amino acid numbers and protease cleavage sites. (B) MVA/S (carrying S-2P), MVA/SdFCS (carrying S-2P and furin-cleavage site-inactivated), and MVA/SdFCS-N (SdFCS with N) vaccines showing the spike expression and ACE2 binding in infected-DF-1 cells by flow cytometry. (C) MVA/S, MVA/SdFCS and MVA/SdFCS-N vaccines showing the spike and N expression in infected-DF-1 cells in Western blotting analysis.