Abstract
Ginger (Zingiber officinale) is considered as a nutraceutical spice, which possesses several health promotion and benefits. This study was carried out to investigate the phyto-chemical composition, the antioxidant capacities, the drug-likeness, and pharmacokinetic properties of ginger extract on kidney injury-associated osteoporosis in rats. Phenolic and flavonoid contents were assessed by standard chemical analysis methods and HPLC. In vivo protective effect was based on the use of female rats to evaluate the effect on renal injury as a result of combined osteoporosis using biochemical markers, oxidative status, and histological analyses. Results showed that ZO contained appreciable amounts of phenolics and flavonoids and it exhibited high scavenging activity. Ovariectomy-associated corticotherapy induced severe renal injury marked by altered biochemical markers (creatinine, urea, and uric acid), reduced GFR, significative oxidative damage signs, and disrupted antioxidant status in the combined osteoporotic rats. The histopathological examination revealed structural modifications of kidney tissues. However, all these changes were reversed following the use of ZO. These results confirm the renoprotective and antioxidant potential of ginger against renal injuries in osteoporotic rats.
Keywords: Drug-likeness, Ginger, Histopathology, Kidney function, Pharmacokinetics
Introduction
An imbalance between resorption and bone formation could occur under pathological conditions, mainly in the case of osteoporosis. This metabolic pathology is characterized by a reduced skeletal mass associated with microarchitectural disruption of the bone (Li et al. 2022; Badraoui et al. 2017; Scarano et al. 2016). This could lead to increase of both fragility and risk of fracture (Consensus development conference 1993). Osteoporosis has been reported to be associated with disturbances in bone metabolism and sex hormones. Reduced bone mass was observed in women during menopause due to increased bone resorption phenomenon. This reduction in bone mass, due to menstrual disturbances, was correlated with renal failure (Díaz López et al. 2003). Likewise, kidney tissue appears to be affected by ovariectomy (OVX). The removal of the ovaries reduced estrogen levels and increased the incidence of various types of cardiovascular and renal disorders (Sayakhot et al. 2011). The risk of developing a renal disease increases essentially after menopause (Mercantepe et al. 2016). Several renal diseases may be considered as consequence of menopause-related oxidative damage. Numerous studies have shown that estrogen deficiency was closely related to oxidative stress (Badraoui et al. 2007, 2010; Selli et al. 2016). Because of the effect of reactive oxygen species (ROS) on mesangial and endothelial cells, changes in both morphological structure and function of the glomeruli were detected (Badraoui et al. 2012).
Many therapies for osteoporosis have presented certain risks to women's health (Badraoui et al. 2017). For this reason, investigators have developed alternative therapies with less undesirable side effects. Based on the findings of previous studies, this trend resulted in increasing demand for the use of nutraceuticals as treatment for various illnesses (Mzid et al. 2017; Zammel et al. 2018). Ginger is one of the traditional medicinal plants that have been used for over 200 years as treatments of several diseases. In Ayurveda literature, Zingiber officinale was obviously used to improve digestion and alleviate constipation and hemorrhoids. ZO was also used for as a cardio-protector, analgesic, and antipyretic agent (Dissanayake et al. 2020). It has been used to treat arthritis, nausea (Mulia and Wulandari 2021), inflammation (Anosike et al. 2009; Zammel et al. 2021b), and hepatotoxicity (Vipin et al. 2017). This spice has been reported to be effective against nephrotoxicity (Lakshmi and Sudhakar 2010). Previous studies sowed that ginger is rich in bioactive substances, which display numerous biological and pharmacological activities such as antioxidant, anti-inflammatory, and anti-carcinogenic (Dugasani et al. 2010; Shukla and Singh 2007; Zammel et al. 2021b). Mahady et al. (2005) reported that ZO was traditionally used as treatment for gastro-intestinal disorders. Recently, Gabr et al. (2019) reported that the aqueous extract of ZO is able to protect kidneys against cadmium-associated nephrotoxicity. Like other traditional herbal medicines, ZO has been used alone or in combination with others herbs for the treatment of osteoporosis (Sakamoto et al. 2000). It could prevent bone loss and vertebral injury in OVX rats as shown by Sassa et al. (2001) and Zammel et al. (2018). Yijung-tang, a traditional Asian medicine, which contains ZO and others spices, was suggested to inhibit the osteoclastogenesis (Kim et al. 2013). There are still few studies investigating the effect of estrogen deficiency and corticotherapy on renal dysfunction. To the best of our knowledge, the effect of ginger on the kidney redox system has not yet been studied on OVX rats treated with glucocorticoids (GCs). The aim of the present investigation was to assess the potential renoprotective and antioxidant activities in a well-reproducible rat combined model of osteoporosis due to gonadal hormone deficiency associated corticotherapy.
Materials and methods
Plant material
Rhizomes of ginger were procured from a local market in Sfax (Tunisia). The rhizomes were washed twice with water to make them soil free and dried at room temperature. Then, they were crushed into a fine powder and dissolved in distilled water and boiled for 10 min, and cooled and filtered using Whatman No.1 filter paper to obtain the aqueous extract.
Estimation of total phenolic content
Total polyphenols’ content was assessed by a widely used method, Folin–Ciocalteu assay (Singleton and Rossi 1965). An aliquot of the extract (50 µL) was mixed with 250 μL of Folin–Ciocalteu reagent and then with 500 μL of sodium carbonate (Na2CO3, 20%). The final volume of the solution was adjusted to 5 mL with distilled water. The mixture was incubated for 30 min at room temperature, and then, the absorbance was recorded at 740 nm using spectrophotometer. Gallic acid was used as standard and the total phenol content was determined according to the following equation:
The total phenol level was expressed in mg of gallic acid equivalents (GAE)/g of dry extract. The given values represent the average of three independent measurements.
Determination of total flavonoids’ content
The concentration of flavonoids in the ZO extract was determined by an aluminum trichloride colorimetric assay, which was described by Zhishen (1999). This method is based on the use of a standard solution of quercetin. 1 mL of the ZO or quercetin extract was added to 4 mL of distilled water in the presence of 300 μL of sodium nitrite solution NaNO2 (5%). After 5 min, 300 μL of AlCl3 (10%) were added. 1 min later, the reaction mixture was added with 2 mL of NaOH (1 M). The final volume was adjusted to 10 mL with distilled water and the absorbance was read at 510 nm. The concentration of total flavonoids was assessed from the quercetin regression equation:
Total flavonoid content was presented as mg of quercetin equivalents (QE)/g of dry extract. The given values represent the average of three measurements.
Antioxidant activity using DPPH method
The diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of the aqueous extract was assessed according to Ebrahimzadeh et al. (2008) with slight modifications. The principle of this test is to measure the capacity of the antioxidants contained in the extract to reduce the stable radical DPPH (purple color) to DPPH-H (yellow color) following the transfer of hydrogen. This discoloration is proportional to the antioxidant activity of the extract. The reaction mixture contains 2 mL of the ZO extract and 2 mL of the DPPH solution (100 μM) in ethanol. The mixture was incubated in a dark area for 30 min at room temperature. Ascorbic acid was used as a standard. The reduction of the free radical DPPH• was measured spectrophotometrically at 517 nm. The percent inhibition (PI) of radicals was estimated using the following formula:
where A control is the absorbance of the blank reaction without the extract sample, and A sample is the absorbance of the sample in which the reagent reaction includes the extract.
HPLC analysis
The identification of phenolic compounds of ZO aqueous extract was carried out using HPLC system (consisting of a vacuum degasser, an auto sampler, and a binary pump with a maximum pressure of 400 bar; Agilent 1260, Agilent technologies, Germany) equipped with a reversed phase C18 analytical column of 4.6 × 100 mm and 3.5 μm particle size (Zorbax Eclipse XDB C18). The DAD detector was set to a scanning range of 200–400 nm. Column temperature was maintained at 25 °C. The injected sample volume was 2 μL and the flow-rate of mobile phase was 0.4 mL/min. Mobile phase B was milli-Q water consisted of 0.1% formic acid and mobile phase A was Methanol. The optimized gradient elution was illustrated as follows: 0–5 min, 10–20% A; 5–10 min, 20–30% A; 10–15 min, 30–50% A; 15–20 min, 50–70% A; 20–25 min, 70–90% A; 25–30 min, 90–50% A; 30–35 min, return to the initial conditions. Identification analysis was performed by comparison of their retention time with those obtained from the extract. For the quantitative analysis, a calibration curve was obtained by plotting the peak area against different concentrations for each identified compound at 280 nm.
Drug-likeness and pharmacokinetics of ZO compounds
Prediction of the drug-likeness and pharmacokinetics of different identified compounds of ZO was performed based on the ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties as previously described (Badraoui et al. 2021; Hchicha et al. 2021; Mhadhbi et al. 2022). Bioavailability of ZO identified compounds was also assessed based on the physico-chemical structures (Badraoui et al. 2021).
Ethics statement
All the animal experiences were conducted following the Guidelines for Care and Use of Laboratory Animals of Sfax University. Rats were sacrificed using thiopental anesthesia and all possible efforts were made to reduce the animal suffering. The experimental procedure was approved by the local ethical committee (Medicine Faculty of Sfax, 12/ES/15–2018).
Animal material and experimental design
Adult female Wistar rats (180–200 g in weight), aged from 8 to 10 weeks, were housed in metal cages at 23 ± 2 °C and a light dark cycle of 12/12 h. The rats were left to acclimatize for 1 week before starting the experimental procedure. Animals were fed with a commercial balanced diet (SICO, Sfax, Tunisia), and provided water ad libitium. Rats were randomly divided into 4 groups each of 6 rats as follows:
Group 1: Animals were injected intraperitoneally with physiological saline solution. This group served as a control group (CTRL).
Group 2: Animals for which we performed bilateral ovarian ablation (OVX) and subjected to intramuscular GC (Cortisol) injections by receiving daily doses of 100 mg/kg of body weight. This group represents a well-reproducible combined model of osteoporosis (CMO) as previously published by Badraoui et al. (2017) and Zammel et al. (2018, 2021a).
Group 3: Ovariectomized and GC-treated rats, as above, received an aqueous extract of ginger (33.33 mg/kg of body weight) by gavage (CMO + ZO). This group will permit to assess the potential effects of this spice against kidney disorders as a result of severe osteopenia.
Group 4: Ovariectomized and GC-treated rats, as above, received daily intake of calperos (3.16 mg/kg of body weight) by gavage (CMO + Clp). Calperos (Clp) was used as referenced medicine as it is commonly prescribed in post-menopause osteoporosis.
The treatment was carried out for 2 months. Dose, duration period, and route of treatment were selected according to previous published reports (Zammel et al. 2018, 2021a). The selected dose was proven to have a potential preventive effect against osteoporosis (Zammel et al. 2018, 2021a).
After 2 months, all rats were sacrificed by cervical decapitation. 24 h before sacrifice, rats were kept in metabolic cages singly to collect urine samples. Specimens were stored at – 20 °C until used for biochemical study. At sacrifice day, blood samples were collected and serum was separated by centrifugation (4000 tours/ min for 15 min) and stored at – 80 °C until analyzed. The kidneys were dissected out, some of them were immediately fixed in 10% neutral formol saline for histopathological investigations, and others were used for oxidative stress assessment.
Biochemical markers in serum and urine
Creatinine, urea, and uric acid were assessed in serum and urine samples using commercial reagent kits (Biomaghreb-Tunis, Tunisia). The kits were used as described by the manufacturer’s instructions.
Glomerular filtration rate (GFR), as an index of kidney function, was evaluated by calculating creatinine and blood urea nitrogen (BUN) clearance, using serum and urine creatinine and BUN concentrations and the urine volume:
Creatinine clearance = (Urine Volume × Concentration of Creatinine in urine) / (Concentration of creatinine in serum × Time).
BUN clearance = (Urine Volume × Concentration of BUN in urine) / (Concentration of BUN in serum × Time),
where Urine Volume (L), Urine concentration of Creatinine and of BUN (mmol/L), Serum concentration of Creatinine and BUN (mmol/L) and Time (min): 1440 = 24 × 60 (min).
GFR = mean of (Creatinine clearance, BUN clearance).
Oxidative stress testing
Protein content in kidneys was determined using bovine serum albumin (standard) as reported in the method of Lowry et al. (1951).
MDA quantification
Lipid peroxidation was determined on the kidney homogenate following the method of Draper and Hadley (1990) by measuring thiobarbituric acid reactive substances (TBARS). Results were presented in terms of malonaldehyde (MDA) levels. This detection is based on a reaction in which one MDA molecule reacts with two molecules of thiobarbituric acid (TBA) resulting in the formation of a red chromogen. An aliquot of kidney extract supernatant was mixed with 1 mL of trichloroacetic acid (TCA, 5%) followed by centrifugation (4000 × g) for 10 min. 500 μL of supernatant was collected and mixed with 1 mL of thiobarbituric acid reagent (TBA, 0.67%). The mixture was heated for 15 min at 95 °C then cooled. MDA content was measured spectrophotometrically at 532 nm. MDA level was expressed in nmoles/mg of tissue.
AOPP level measurements
Spectrophotometric determination of advanced oxidation protein products (AOPP) was performed following the method of Kayali et al. (2006). The reaction mixture contained 1600 μL of phosphate buffer (0.1 M PBS; pH = 7.4), and 400 μL and 100 μL of potassium iodide (1.16 M KI). After 2 min of incubation, 200 μL of acetic acid was added. Chloramine-T was used as standard and the absorbance was read at 340 nm, and the AOPP results were expressed as µmoles of AOPP/mg of protein.
Determination of antioxidant activities in kidney tissues
Determination of superoxide dismutase (SOD) activity was effected as previously published (Beauchamp and Fridovich 1971). One unit of SOD activity was determined as the amount of enzyme that inhibited 50% of the NBT reduction. The reaction product was assessed spectrophotometrically at 560 nm. The SOD activity was expressed as units/mg of protein.
Catalase (CAT) activity was evaluated following the decrease of the absorbance at 240 nm as a result of the enzymatic decomposition of H2O2 (Aebi 1984). The enzyme activity was given as μmoles of H2O2 consumed/min/mg of protein.
Glutathione peroxidase (GPx) activity was measured according to the method described by Flohe and Gunzler (1984). The principle of this method is based on the conversion of the oxidized reduced glutathione to the reduced form with a concomitant oxidation of NADPH–NADP+. The decrease of absorbance was measured spectrophotometrically at 340 nm. GPx activity was expressed as nmoles of GSH oxidized/min/mg protein.
Glutathione (GSH) levels were determined according to Ellman et al. (1959) modified by Jollow et al. (1974). The method was based on the changes in absorbance resulting from the conversion of NADPH into NADP. The absorbance was measured at 412 nm and the results were expressed as μg/g tissue.
Histopathological examination
The kidney samples, serving for the histological examination, were immediately fixed to avoid any tissue autolysis (10% formalin solution). The specimens were processed for standard histological procedure, dehydrated in ascending series of alcohol, and then embedded in paraffin. Sections of 5 μm thickness were subjected to hematoxylin and eosin stain (H&E) (Gabe 1968).
Statistical analysis
Statistical analyses were performed using the SPSS software package. Data were expressed as Mean ± SEM. The one-way analysis of variance (ANOVA) was performed and followed by Tukey post hoc test for comparison between groups. Differences with p < 0.05 were considered statistically significant.
Results
Total phenolic and flavonoids contents’ determination
The estimation of phenolic content revealed that the aqueous extract of ZO contained 95.376 ± 4.162 mg GAE/g dry extract. The flavonoids level was found to be around 230.370 ± 76.918 mg QE/g dry extract (Table 1).
Table 1.
Chemical composition of ginger aqueous extract
| Extract | Phenolic contents (mg GAE/g dry extract) | Flavonoids’ contents (mg QE/g dry extract) |
|---|---|---|
| ZO aqueous extract | 95.37 ± 4.16 | 230.37 ± 76.91 |
Values are expressed as mean ± SEM of three independent determinations
ZO Zingiber officinale, GAE gallic acid equivalent, QE quercetin equivalent
Antiradical activity (DPPH)
Zingiber officinale was tested for its radical scavenging property (DPPH assay) (Table 2). The aqueous extract of ZO and the used standard (Vit C) showed a powerful antioxidant potential of 71% and 76%, respectively, at a concentration of 2.5 mg/mL. Indeed, the tested extract was able to reduce 50% of this radical at a concentration (IC50) of 0.35 ± 0.004 mg/mL. Our results showed that IC50 of DPPH of the aqueous extract of ZO was significantly near to that of the ascorbic acid (0.146 ± 0.003 mg/mL).
Table 2.
DPPH-radical scavenging activities of ginger aqueous extract
| Assay | Vitamin C | ZO aqueous extract |
|---|---|---|
| DPPH IC50 (mg/mL) | 0.146 ± 0.03 | 0.35 ± 0.004 |
Values are expressed as mean ± SEM of three independent determinations
ZO Zingiber officinale, DPPH diphenyl-2-picrylhydrazyl, IC50 50% Inhibition concentration
HPLC findings
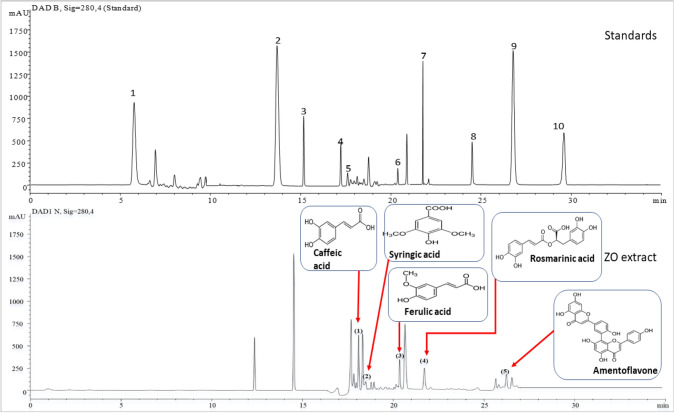
Figure 1 shows the main results of the HPLC analysis of ginger. The evaluation of the obtained phenolic profile resulted in the identification of five phenolic compounds. In terms of their quantities, these compounds were as follows; caffeic acid, ferulic acid, rosmarinic acid, amentoflavone, and syringic acid. The 1st peak, eluted at 17.23 min, indicated that the identified compound is caffeic acid. The 2nd peak (TR = 17.63 min) corresponded to syringic acid. The 3rd peak (TR = 20.27 min) was identified as ferulic acid. The corresponding compound identified from the 4th peak (TR = 21.78 min) was rosmarinic acid and the 5th peak (TR = 26.94 min) was assigned to amentoflavone.
Fig. 1.
HPLC Chromatographic profiles of standard phenolic compounds and ZO extract. The peak numbers assigned to: (1) caffeic acid; (2) syringic acid; (3) ferulic acid; (4) rosmarinic acid; and (5) amentoflavone
Drug-likeness and pharmacokinetics of ZO phyto-chemical compounds
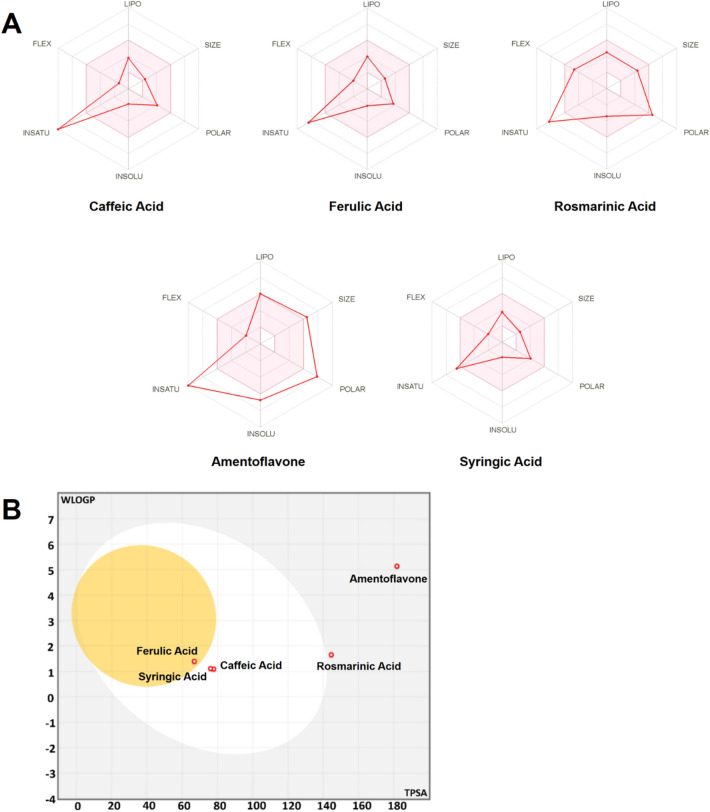
The drug-likeness and pharmacokinetics properties of the ZO identified compounds are presented in Table 3. These parameters are the main criteria used for screening new drugs and avoid any possible drug failure during the clinical phase at the early steps of the drug discovery process. Regardless amentoflavone, all the compounds were found to meet Lipinski’s rule of five without any violation. Another criterion which based on a probability value of a molecule to have an optimum profile of bioavailability is the bioavailability score (BAS). The bioavailability score of the assessed compounds varied between 0.17 and 0.85, which indicates their feasibility to be administered orally. The bioavailability radars (Fig. 2A) exhibited the suitability of the assessed compounds for oral bioavailability. While only ferulic acid was found to be Blood–Brain Barrier (BBB) permeant, none of the phyto-chemical compounds was P-gp substrate. Cytochrome P450 enzymes play a major role in drug interaction, metabolism, and excretion inside the body. From 57 CYP isoforms, five CYPs (CYPs 3A4, 2D6, 2C19, 2C9, and 1A2) are responsible for the metabolism of more than 80% of clinically used drugs. Inhibition of these enzymes may lead to diminishing metabolism of the drug resulting in high probability of adverse toxicological outcomes. Furthermore, none of the compounds was predicted to inhibit the Cytochrome P targeted isomers (CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4). P-glycoproteins facilitate the transport of drugs, and therefore, their inhibition may affect the normal drug transport. High gastro-intestinal (GI) absorption was found with caffeic, ferulic, and syringic acids. The boiled-egg model (Brain Or IntestinaL EstimateD permeation model) confirmed these findings (Fig. 2B). The white region represented the physico-chemical space of molecules with highest probability of being absorbed by the GI tract, and the yellow region (yolk) represented the physico-chemical space of molecules with highest probability to permeate to the brain. Regarding skin permeability values (Kp), a more negative values mean lower skin permeability, Kp's skin permeability values of the tested compounds ranged from -6.01 to -6.82 cm/s indicating moderate-to-low skin permeability.
Table 3.
Lipophilicity, drug-likeness, medicinal chemistry, and pharmacokinetics based on ADMET (for absorption, distribution, metabolism, excretion, and toxicity) properties of the major ZO identified compounds
| Entry/compound name | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Caffeic acid | Ferulic acid | Rosmarinic acid | Amentoflavone | Syringic acid | ||
| Lipophilicity/Drug-likeness | Molecular weight | 180.16 | 194.18 | 360.31 | 538.46 | 198.17 |
| TPSA (Å2) | 77.76 | 66.76 | 144.52 | 181.80 | 75.99 | |
| Consensus Log Po/w | 0.93 | 1.36 | 1.58 | 3.62 | 0.99 | |
| Lipinskiˈs Rule | Yes | Yes | Yes | No | Yes | |
| Bioavailability Score | 0.56 | 0.85 | 0.56 | 0.17 | 0.56 | |
| Pharmacokinetics/Medicinal Chemistry | GI absorption | High | High | Low | Low | High |
| BBB permeant | No | Yes | No | No | No | |
| P-gp substrate | No | No | No | No | No | |
| CYP1A2 inhibitor | No | No | No | No | No | |
| CYP2C19 inhibitor | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | |
| CYP3A4 inhibitor | No | No | No | No | No | |
| Log Kp (cm/s) | − 6.58 | − 6.41 | − 6.82 | − 6.01 | − 6.77 | |
| Leadlikeness | No | No | No | No | No | |
| Synthetic accessibility | 1.81 | 1.93 | 3.38 | 4.27 | 1.70 |
Fig. 2.
Bioavailability radars (A) and boiled-egg model (plot of WLOGP against TPSA, B) for the major ZO identified compounds. LIPO lipophilicity, SIZE Molecular size, POLAR Polarity, INSOLU Insolubility, INSATU Insaturation, FLEX Flexibility. The pink-colored area in the bioavailability radars corresponds to the most suitable zone for oral bioavailability. The white- and yellow-colored areas in the boiled-egg model correspond to the highest probability of GI absorption and BBB permeation according to the physico-chemical structures, respectively
Body and organ weight
The effect of osteoporosis, due to OVX-associated corticotherapy, and ginger administration on the body and the kidney weights as well as the relative weight of the studied animals are shown in Table 4. In CMO group, the body weight was significantly increased (p < 0.01) and the kidney weight was decreased (p < 0.01) once compared with the CTRL rats. However, rats treated either with ZO or Clp (p < 0.01) exhibited normal ranges of both body and kidney weight as those of the CTRL group. Decreased relative weight was highly significant (p < 0.01) in CMO group when compared to CTRL group. In comparison with the osteoporotic group, this parameter was significantly increased in rats treated with ZO or Clp (Table 4).
Table 4.
Body, kidney, and relative weights of the different groups: control (CTRL), combined model of osteoporosis (CMO), and osteoporotic rats treated with ZO (CMO + ZO) or with Clp (CMO + Clp)
| Parameters | CTRL | CMO | CMO + ZO | CMO + Clp |
|---|---|---|---|---|
| Body Weight (g) | 199.62 ± 2.62 | 235.5 ± 4.21** | 206.35 ± 2.56## | 200 ± 1.78## |
| Kidney Weight (g) | 1.41 ± 0.04 | 1.12 ± 0.04** | 1.34 ± 0.03## | 1.28 ± 0.04## |
| Relative Weight | 0.70 ± 0.01 | 0.477 ± 0.01** | 0.65 ± 0.02## | 0.62 ± 0.02*## |
The data represent the mean ± SEM. Comparisons are made between two groups: Osteoporotic (CMO) versus control group (CTRL): *p < 0.05; **p < 0.01; Osteoporotic treated with ZO (CMO + ZO) or Clp (CMO + Clp) versus osteoporotic group (CMO): #p < 0.05; ##p < 0.01. ZO Zingiber officinale, Clp Calperos
Biochemical findings
As shown in Table 5, osteoporotic rats presented significant (p < 0.01) higher serum biochemical markers (creatinine, urea, and uric acid) once compared with CTRL rats. However, the administration of either ZO or Clp to rats restored back the levels of those markers to near normalcy. Severe osteopenia (CMO group) significantly reduced creatinine and urea levels and increased uric acid in urine samples (p < 0.05). In groups treated with ZO or Clp, significant increases (p < 0.01) in creatinine and urea values were observed, while uric acid level was found to be reduced (p < 0.05).
Table 5.
The effects of severe osteopenia and ginger on renal biochemical markers in control (CTRL), combined model of osteoporosis (CMO) and osteoporotic rats treated with ZO (CMO + ZO) or with Clp (CMO + Clp)
| Parameters | CTRL | CMO | CMO + ZO | CMO + Clp | |
|---|---|---|---|---|---|
| Serum | Creatinine (µmol/L) | 30.5 ± 0.86 | 36.33 ± 1.08** | 29.83 ± 1.19## | 30.16 ± 1.37## |
| Urea (mmol/L) | 6.15 ± 0.06 | 7.63 ± 0.29** | 6.26 ± 0.15## | 6.73 ± 0.14# | |
| Uric acid (mmol/L) | 67.5 ± 1.32 | 77.83 ± 2.75* | 68.5 ± 1.17# | 68.8 ± 1.30# | |
| Urine | Creatinine (µmol/L) | 9.54 ± 0.56 | 7.25 ± 0.47* | 9.21 ± 0.20## | 8.91 ± 0.40# |
| Urea (mmol/L) | 1359 ± 146.24 | 978 ± 28.40* | 1324 ± 108.58# | 1322 ± 120.09# | |
| Uric acid (mmol/L) | 618 ± 42.47 | 889 ± 86.96* | 641.75 ± 46.08# | 612 ± 110.70 |
The data represent the mean ± SEM. Comparisons are made between two groups: Osteoporotic (CMO) versus control group (CTRL): *p < 0.05; **p < 0.01. Osteoporotic treated with ZO (CMO + ZO) or Clp (CMO + Clp) versus osteoporotic group (CMO): #p < 0.05; ##p < 0.01. ZO Zingiber officinale, Clp Calperos
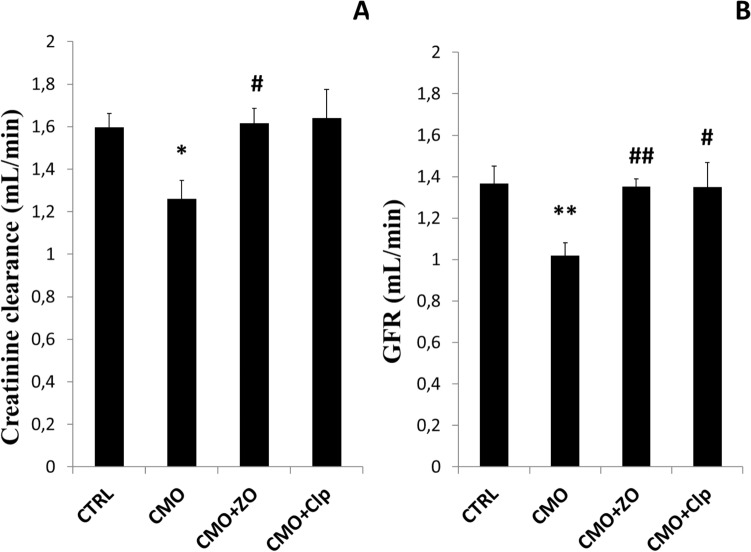
The effect of OVX and GCs on creatinine clearance and GFR is shown in Fig. 3. Significant reduces of creatinine clearance (Fig. 3A) and GFR (Fig. 3B) were observed in CMO group when compared to CTRL. The use of either ZO or Clp tended to mitigate these disruptions in treated groups (CMO + ZO and CMO + Clp).
Fig. 3.
The effect of severe osteopenia and ginger on creatinine clearance and GFR in control (CTRL), combined model of osteoporosis (CMO) and osteoporotic treated rats with ZO (CMO + ZO) or with Clp (CMO + Clp). The data represent the mean ± SEM. Comparisons are made between two groups: Osteoporotic (CMO) versus control group (CTRL): *p < 0.05; **p < 0.01; Osteoporotic treated with ZO (CMO + ZO) or Clp (CMO + Clp) versus osteoporotic group (CMO): # p < 0.05; ## p < 0.01
Oxidative stress parameters
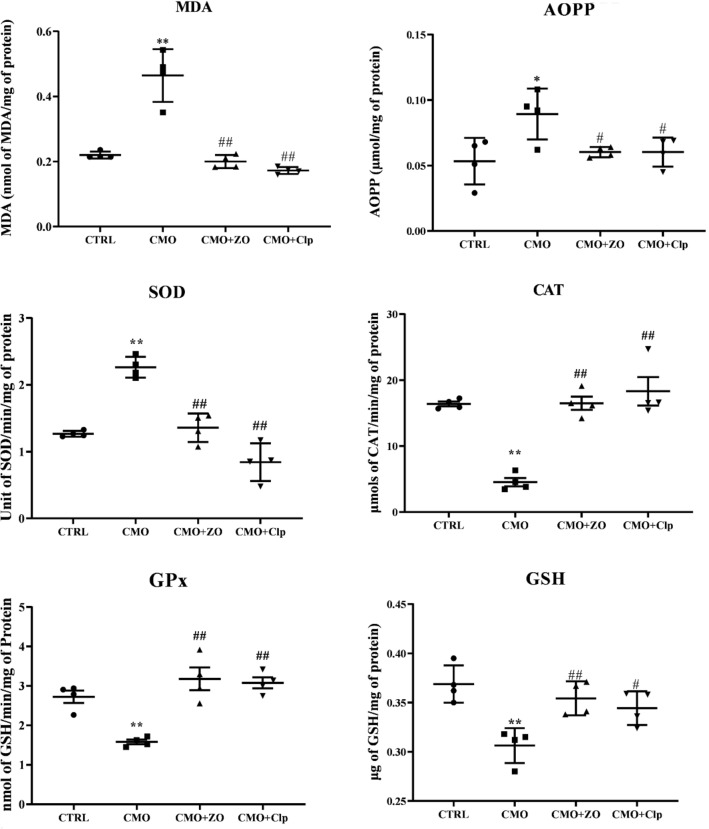
Figure 4 shows the influence of osteoporosis and ginger administration on oxidative stress and antioxidant defense system on rat’s renal tissues. Ovariectomy-associated GCs resulted in a significant increase in AOPP (p < 0.05, Fig. 4A) and MDA (p < 0.01, Fig. 4B) levels in the kidney tissues of CMO rats when compared to CTRL. However, treated groups with either ZO or Clp have significantly decreased MDA and AOPP (p < 0.01 and p < 0.05, respectively) contents as compared with CMO group (osteoporotic group). Furthermore, CMO group exhibited a highly significant increase (p < 0.01) of SOD activity in comparison with the CTRL group (Fig. 4C). ZO-treated group and Clp-treated group showed a significant decrease (p < 0.05) in the SOD activity when compared with untreated group (CMO). SOD activity in treated groups was similar to that of the CTRL group. Conversely, both GPx (Fig. 4D) and CAT (Fig. 4F) activities exhibited a remarkably decrease (p < 0.01) in CMO group when compared to CTRL. Treatment with ginger improved significantly (p < 0.01) the activities of those antioxidant enzymes. Similar results were observed in the CMO + Clp group. Compared to CTRL rats, renal GSH content was significantly decreased in CMO rats. ZO or Clp treatment restored renal GSH levels to normalcy (Fig. 4E).
Fig. 4.
The effect of severe osteopenia and ginger on renal protein and lipid peroxidation, superoxide dismutase, catalase and glutathione peroxidase activities and GSH content (AOPP, MDA, SOD, CAT, GPx, and GSH, respectively), in control (CTRL), combined model of osteoporosis (CMO) and osteoporotic treated rats with ZO (CMO + ZO) or with Clp (CMO + Clp). The data represent the mean ± SEM. Comparisons are made between two groups: Osteoporotic (CMO) versus control group (CTRL): *p < 0.05; **p < 0.01; Osteoporotic treated with ZO (CMO + ZO) or Clp (CMO + Clp) versus osteoporotic group (CMO): # p < 0.05; ## p < 0.01
Histological findings
Histological assessments of kidney slides of all experimental groups are presented in Fig. 5. Normal morphological structures of renal tissue were seen in the CTRL group (Fig. 5A). However severe osteopenia resulted in moderate and massive glomerular and renal tubular damages. The histomorphology of kidney showed pseudo-lobulated glomeruli with tubular epithelial loss, desquamation, and necrosis (Fig. 5B and 5B’). Moreover, diffused hemorrhage areas, leukocyte infiltration, and excess of cell debris in the tubular lumen were shown. Treatments with ZO (Fig. 5C) or with Clp (Fig. 5D) were found to reduce these morphological changes induced by osteoporosis. However, hemorrhage and a little cell debris features were seen.
Fig. 5.
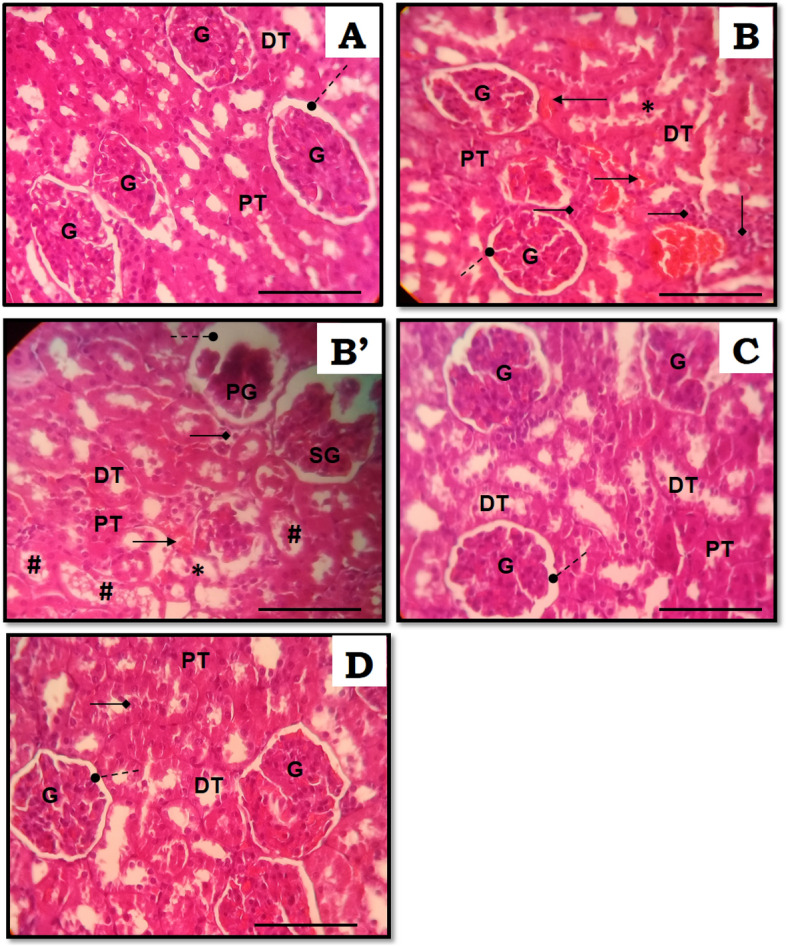
Kidney histological photographs of rats stained with hematoxylin–eosin (HE) of controls and experimental groups. (A) CTRL rats showed normal appearance of kidney, normal glomeruli and tubules. (B and B’) CMO rats showed multiple foci of hemorrhage and necrosis. (C + D) Showed almost normal cellular architecture of the kidney in CMO + ZO and CMO + Clp rats, respectively. Original magnification × 400 (scale bar 0.1 mm). G glomerulus, DT distal tubule, PT proximal tubule, PG pseudo-lobulated glomerulus, SG shrinked glomerulus, #Necrotic debris in tubular lumen; *Necrosis; Arrows indicate: (filled circle) Bowman’s space (filled diamond) Leukocyte infiltration (right arrow) Hemorrhage
Discussion
This study aimed to evaluate the effect of severe osteopenia on renal injuries and oxidative status in rats and the protective potential of ginger against these disturbances. Sex hormones, like estradiol, are involved in the maintenance of kidney functions (Ahmed and Ramesh 2016). However, the decrease in the levels of those hormones could alter both the kidney’s structure and function. In the present study, disruptions in renal serum markers were noted in CMO group when compared to CTRL rats. These results may be due to the effect of the severe osteopenia and the high osteoclast resorbing activity, which has been previously reported (Badraoui et al. 2017; Zammel et al. 2018). Thus, the severe osteopenia may have a direct effect on the kidney function. OVX-associated corticotherapy altered renal function, which is manifested by a significant decrease in creatinine clearance and glomerular filtration rate. It has been reported that it might be due to the lack of anabolic effect of estrogens. Decreased creatinine clearance and decreased GFR may be the cause of this elevation (Ratliff et al. 2016). It was outlined that the rises in uric acid levels, which have been implicated on the kidney pathogenesis, were associated with renal histopathological features. Acute renal failure may increase uric acid levels by reducing renal excretion. Several studies reported that, following an overproduction of uric acid, renal failure may occur (Mzid et al. 2017; Badraoui et al. 2012; Amri et al. 2018; Rahmouni et al. 2019).
Enhanced uric acid levels are seen in patients presenting low GFR values. GFR, which is considered as an important parameter for the comprehension of the mechanistic regulation in kidney tissue (Ejaz et al. 2007), was assessed using serum creatinine data (Inker et al. 2012). In our experimental study, the estimation of GFR indicated that severe osteopenia caused by both estrogen deficiency and corticosteroids affected renal function in CMO rats. Physiological loss of nephrons and decreased GFR were seen with the progressive aging. Lower GFR was reported to be correlated with reduced kidney function. GFR and creatinine clearance tend to fall in aging and in the case of bone diseases including osteoporosis. Estrogens appeared to have protective role against the development of focal and segmental glomerulosclerosis (FSGS). This confirmed that OVX contributes to kidney disease progression and FSGS acceleration in animal models (Liu et al. 2021). Recently, a study suggested that the association of high GFR levels with kidney damage indicated renal dysfunction (Fiebiger et al. 2020).
Glucocorticoids could influence kidney development as well as glomerular and tubular function in mature kidneys usually on dose- and time-dependent manner (Mangos et al. 2003; Bailey et al. 2009). Long treatment with GCs may induce alterations in GFR (Smets et al. 2010). Contrary to our obtained data, the previous studies reported rise in GFR in human and rats treated with GCs alone (Smets et al., 2010). Several studies showed that GCs were prescribed for the treatment of renal impairment (Zhang et al. 2013).
OVX plays a crucial role in the progression of some renal pathological alterations through heavy production of ROS, which may lead to an eventual kidney failure. Oxidative stress occurs when an imbalance debarks, between pro-oxidants and their elimination by antioxidants (Badraoui et al. 2007; Amri et al. 2017). Oxidative stress is evidenced by the increased MDA and AOPP levels in damaged tissue (Amri et al. 2017; Akacha et al. 2020; Mzid et al. 2017). Under our experimental conditions, MDA and AOPP levels were significantly elevated in the kidneys of CMO rats compared to CTRL. Our results confirmed the previous studies, which demonstrated that increased MDA and AOPP levels are linked to increased ROS production (Amri et al. 2017; Badraoui et al. 2007; Mzid et al. 2017). Previously, the lack of estrogens was related to increased oxidative stress markers. It enhanced lipid peroxidation in various organs, including bone (Chung et al. 2021), kidney, and liver (Amri et al. 2018; Rakshit et al. 2021). OVX was associated with increased pro-oxidants and decreased antioxidants levels (Konyalioglu et al. 2007) in various tissues including the bone (Almeida et al. 2007).
Investigators have suggested that OVX increases oxidative stress, thereby impairing kidney function (Liu et al. 2021). The kidney might receive too much load following the osteolysis inducing hypercalcemia (Badraoui et al. 2017). Furthermore, renal tubular proliferation and renal fibrosis were described as consequences of ROS accumulation resulting from oxidative stress (Badraoui et al. 2007; Sanz et al. 2011; Zammel et al. 2021b), which is well linked to the loss of renal function (Podkowińska and Formanowicz 2020) by inducing apoptosis of podocytes (Xing et al. 2021) and negatively affecting the tightness of the glomerular filtration barrier (Sverrisson et al. 2015). This may be exacerbated by GC treatment. It has been shown that GCs through the overproduction of ROS affected the redox physiology (Lin et al. 2004; Fontella et al. 2005).
Excessive ROS led to the weakening of the antioxidant system. Serious investigations reported the ability of antioxidant enzymes to cleanse ROS radicals in kidney cells (Tang et al. 2012; Amri et al. 2017; Zahedi et al. 2015). Based on our results, the antioxidant defense system was influenced by both OVX and GCs. SOD activity was significantly increased in stressed animals’ kidneys, whereas CAT and GPx activities were reduced (CMO group).
Estrogen deficiency in post-menopausal patients was in correlation with oxidative stress development (Cervellati et al. 2014). This study is in accord with the other studies, which emphasized that estrogen deficiency induced oxidative damage and had inhibitory effect on CAT and GPx activities. Increased MDA levels and decreased CAT and GPx activities were found in osteoporotic women compared to healthy ones (Ozgocmen et al. 2007). Sexual hormones boosted the expression of SOD, CAT, and GPx across the antioxidant response element signaling pathway (Halliwell and Gutteridge 2015). As the most abundant intracellular antioxidant, GSH is used as substrate for GPx. The present results showed that the osteoporotic group had decreased GSH levels as well as GPx activity.
The antioxidant capacity of tissues was reduced by GCs (McIntosh et al. 1998). Long treatment with dexamethasone could induce the depletion of antioxidant enzymes (You et al. 2009; Bera et al. 2010). The increased SOD activity observed in the current study could be explained by the effect of GCs associated OVX, which have been demonstrated to increase the activity of this enzyme. These findings supported the previous observations that compounds with antioxidant properties, including ginger, could alleviate the renal tissue stress (Yang et al. 2014).
The histopathological findings supported the changes in biochemical markers and oxidative stress parameters. These findings showed that the major damage caused by severe osteopenia occurred, especially glomeruli and proximal tubules. In this study, necrosis of kidney tissue is the key histopathological change in the damaged kidney of the osteoporotic rat. Pseudo-lobulated glomerulus (PG) and shrinked glomerulus (SG) were clearly seen due to glomerular compressing and partial endothelial damage in capsules. Proximal tubules were dilated with loss of cellular boundary and epithelial degeneration. These morphological findings were in accordance with the previous studies, which suggested that decreased production of estrogen caused pathological changes and inflammatory cell infiltration in kidneys (Nissen et al. 2012). Free radicals were also proven to damage the glomerular basement membrane (Bahri et al. 2021). In this context, nutraceuticals that possessed rich phenolic and flavonoid content, such as ZO, may have potential ameliorative effects via modulation of immuno-modulators disruptions, oxidative damage, and health promotion.
In our study, ginger administration protected against renal dysfunction induced by severe osteopenia. Rats receiving ZO showed normal levels of renal biomarkers (creatinine, uric acid, and urea) in both serum and urine samples. The use of ZO has reversed the changes on creatinine clearance and GFR. Ginger was shown capable to alleviate these disruptions.
ZO may improve renal function by increasing enzyme antioxidant activities and limiting renal damage caused by the corticosteroid therapy associating gonadal hormone deficiency. Moreover, histological examination showed that ginger markedly reduced the morphological injuries induced by severe osteopenia and improved histopathological architecture of kidney compared to the osteoporotic group.
The present study showed that the harmful effects of sexual hormone deprivation and GCs were reversed by ginger treatment, suggesting an antioxidative/therapeutic potential effect of this plant. These protective effects could be linked to the chemical content of the plant. In fact, the phyto-chemical studies revealed the presence of phenolics and flavonoids, which could contribute to the antioxidant activity of ginger. Similarly to those of Mošovská et al. (2015), our results showed that ZO aqueous extract contained appreciable quantities of phenols and flavonoids. Therefore, this extract displayed powerful antioxidant potentialities. ZO showed high scavenging activity against DPPH radicals. These findings were supported by the previous ones of Stoilova et al. (2007) who signaled that ginger possessed higher radical scavenging activity than quercetin. This antiradical activity is due to the presence of flavonoids and polyphenols (Köksal et al. 2017). Furthermore, a strong correlation was observed between the total phenolic content and antioxidant activity. Antioxidant potentiality was more effective as the levels of those compounds are high. DPPH scavenging activities were linked to their hydrogen donating ability (Ghasemzadeh et al. 2010).
The richness of ginger in polyphenols and flavonoids could enhance renal antioxidant system. In their investigation, Lakshmi and Sudhakar (2010) indicated that ZO extracts possess significant nephroprotective activity. The antioxidant properties of ZO are due to the rich phenolic content. Our HPLC results revealed that ZO aqueous extracts contained rosmarinic acid, caffeic acid, ρ-hydroxybenzoic acid, luteolin, and quercetin. These compounds displayed potential activity against free radicals, and showed promising drug-likeness and pharmacokinetic properties associated the previously reported anti-inflammatory effect (Zammel et al. 2021b; Koleva et al. 2000).
Caffeic acid and Ferulic acid, which are the most abundant in our experimental extract, were involved in the blockage of lipid peroxidation (Göçer and Gülçin 2011; Zammel et al. 2021b). Thanks to their radical scavenging potentialities via hydrogen atom donation, phenolic acids were considered as the most powerful antioxidant compounds. Their potential capacity has been well documented. Previous studies confirmed that caffeic acid restored the altered antioxidant defense system (Gökçe et al. 2009; Gong et al. 2012) and protect kidney against injuries (Akyol et al. 2014). Similarly, ferulic acid has been shown to prevent kidney cell damage induced by oxidative stress (Sanjeev et al. 2019). In nephropathological cases, syringic acid as well as rosmarinic acid exhibited potential role on the improvement of renal histopathological changes (Bayomy et al. 2017; Rashedinia et al. 2020).
The drug-likeness and pharmacokinetics properties of the ZO identified compounds are presented in Table 3. These parameters are the main criteria used for screening new drugs and avoid any possible drug failure during the clinical phase at the early steps of the drug discovery process. Regardless amentoflavone, all the compounds were found to meet Lipinski’s rule of five without any violation (Lipinski et al. 2001). Our results suggested that ginger compounds theoretically meet the. Another criterion which based on a probability value of a molecule to have an optimum profile of bioavailability is the bioavailability score (BAS). The bioavailability score of the assessed compounds varied between 0.17 and 0.85, which indicates their feasibility to be administered orally. The bioavailability radars exhibited that ZO identified compounds are good candidates for oral bioavailability according to their physico-chemical properties.
While only ferulic acid was found to be Blood–Brain Barrier (BBB) permeant, none of the phyto-chemical compounds was P-gp substrate. Cytochrome P450 enzymes play a major role in drug interaction, metabolism, and excretion inside the body. From 57 CYP isoforms, five CYPs (CYPs 3A4, 2D6, 2C19, 2C9, and 1A2) are responsible for the metabolism of more than 80% of clinically used drugs (Banerjee et al. 2020; Das et al. 2020). Inhibition of these enzymes may lead to diminishing metabolism of the drug resulting in high probability of adverse toxicological outcomes (Anzenbacher and Anzenbacherova 2001). Furthermore, none of the compounds was predicted to inhibit the Cytochrome P targeted isomers (CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4). P-glycoproteins facilitate the transport of drugs, and therefore, their inhibition may affect the normal drug transport. High gastro-intestinal (GI) absorption was found with caffeic, ferulic, and syringic acids. The boiled-egg model (Brain Or IntestinaL EstimateD permeation model) (Daina et al. 2017), confirmed these findings (Fig. 2). The white region represented the physico-chemical space of molecules with highest probability of being absorbed by the GI tract, and the yellow region (yolk) represented the physico-chemical space of molecules with highest probability to permeate to the brain (Daina and Zoete 2016). Regarding skin permeability values (Kp), a more negative values mean lower skin permeability (Mishra and Dahima 2019), Kp's skin permeability values of the tested compounds ranged from – 6.01 to – 6.82 cm/s indicating moderate-to-low skin permeability.
Overall, it could be deduced that all ginger phyto-chemical compounds, which have been screened for their ADMET prediction, were considered to be suitable drug-like molecules and possessed acceptable pharmacokinetic properties. This can explain, even in part, the in vivo beneficial effects of ZO extract on the rat model of severe osteopenia. It also confirms the potential nutraceutical effect of ZO and encourage it production as functional food.
Conclusions
In conclusion, our work launches three points. First, gonadal hormone deprivation-associated corticotherapy-induced osteoporosis leads to glomerular and tubular injuries. Furthermore, increases in serum levels of creatinine, urea, and uric acid and decreased creatinine and GFR pointed out a renal dysfunction. The second point is that osteoporosis induced kidney’s complication associated with oxidative damage. The third point we established is that the administration of ginger eliminated functional and structural alterations induced by ovariectomy-associated corticotherapy. In extrapolation, we deduce that osteoporosis has a negative impact on kidney function and that the ameliorative effects of ginger on the kidney alterations might be due to its antioxidant potential, drug-likeness, and pharmacokinetic properties of its phyto-chemical content. The ZO identified compounds can be considered as good candidates for pharmaceutical production of functional foods, which mimic renoprotective effects, which result from severe osteopenia. The major limitation of the study is screening of the total phyto-chemical composition by better analytical technics such as LC–ESI–MS/MS, LC–DAD-MS, and HR-LCMS.
Accession Numbers
None molecular data accession numbers for16S rRNA gene, other rRNA genes, ITS, WGS, SRA etc. or culture collection numbers for new taxa have been used in this work.
Acknowledgements
Authors would like to express their gratitude to Pr. Kamel Jamoussi and Dr. Rim Chaabane for their support. This research received grants from the deanship of scientific research, University of Ha’il. Project number: RG-21 100.
Author contribution
Conceptualization, N.Z., T.R. and R.B.; methodology, N.Z., O.J., and R.B.; Experimental analysis: N.Z., T.R., and R.B; validation, W.S.H., S.E., A.J., and R.B.; formal analysis, N.Z., J.M.A., A.S.E., and O.J.; investigation, R.B.; resources, T.R., and R.B.; data curation, N.Z., A.J.S., H.N., and R.B.; software, N.Z. and R.B.; writing—original draft preparation, N.Z. and R.B.; writing—review and editing, N.Z. and R.B.; visualization, M.M.A.; J.M.A. and R.B.; supervision, T.R.; R.B.; project administration, R.B. All authors have read and agreed to the published version of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest in the publication.
Informed consent
Not applicable for this study.
References
- Aebi H (1984) [13] Catalase in vitro. In: Methods in Enzymology. Academic Press, pp 121–126 [DOI] [PubMed]
- Ahmed SB, Ramesh S. Sex hormones in women with kidney disease. Nephrol Dial Transplant. 2016;31(11):1787–1795. doi: 10.1093/ndt/gfw084. [DOI] [PubMed] [Google Scholar]
- Akacha A, Badraoui R, Rebai T, Zourgui L. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1856187. [DOI] [PubMed] [Google Scholar]
- Akyol S, Ugurcu V, Altuntas A, et al (2014) Caffeic acid phenethyl ester as a protective agent against nephrotoxicity and/or oxidative kidney damage: a detailed systematic review. In: Sci World J https://www.hindawi.com/journals/tswj/2014/561971/. Accessed 24 Dec 2020 [DOI] [PMC free article] [PubMed]
- Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri N, Rahmouni F, Chokri MA, et al. Histological and biochemical biomarkers analysis reveal strong toxicological impacts of pollution in hybrid sparrow (Passer domesticus × Passer hispaniolensis) in southern Tunisia. Envir Sci Poll Res. 2017;24:17845–17852. doi: 10.1007/s11356-017-9352-3. [DOI] [PubMed] [Google Scholar]
- Amri N, Rebai T, Jardak N, Badraoui R. Nephrotoxicity in Hybrid sparrow (Passer domesticus × Passer hispaniolensis) living near a phosphate treatment factory complex in southern Tunisia: a biochemical and histological study. Envir Sci Poll Res 25 (16), 15404–15410. 10.1007/s11356-018-1640-z [DOI] [PubMed]
- Anosike CA, Obidoa O, Ezeanyika LUS, Nwuba MM. Anti-inflammatory and anti-ulcerogenic activity of the ethanol extract of ginger (Zingiber officinale) Afr J Biochem Res. 2009;3:379–384. doi: 10.5897/AJBR.9000025. [DOI] [Google Scholar]
- Anzenbacher P, Anzenbacherova E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001;58:737–747. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badraoui R, Sahnoun Z, Abdelmoula NB, et al. May antioxidants status depletion by Tetradifon induce secondary genotoxicity in female Wistar rats via oxidative stress? Pestic Biochem Physiol. 2007;88:149–155. doi: 10.1016/j.pestbp.2006.10.007. [DOI] [Google Scholar]
- Badraoui R, Abdelmoula NB, Feki N, et al. Endocrine disruption and ovarian morphometric responses in rats following exposure to tetradifon. Gen Comprat Endocrinol. 2010;166:268–272. doi: 10.1016/j.ygcen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Badraoui R, Nasr HB, Louati R, et al. Nephrotoxic effect of tetradifon in rats: A biochemical and histomorphometric study. Exp Toxicol Pathol. 2012;64:645–650. doi: 10.1016/j.etp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Badraoui R, Amri N, Zammel N, et al. Corticosteroid treatment exacerbates bone osteopenia in mice with gonadal hormone deficiency-induced osteoporosis. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2017;105:41–46. doi: 10.1016/j.ejps.2017.04.023. [DOI] [PubMed] [Google Scholar]
- Badraoui R, Adnan M, Bardakci F, et al. Chloroquine and hydroxychloroquine interact differently with ACE2 domains reported to bind with the coronavirus spike protein: mediation by ACE2 polymorphism. Molecules. 2021;26:673. doi: 10.3390/molecules26030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- Bailey MA, Mullins JJ. Kenyon CJ (2009) Mineralocorticoid and glucocorticoid receptors stimulate epithelial sodium channel activity in a mouse model of Cushing syndrome. Hypertens Dallas Tex. 1979;54:890–896. doi: 10.1161/HYPERTENSIONAHA.109.134973. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Dunkel M, Kemmler E, Preissner R. SuperCYPsPred—a web server for the prediction of cytochrome activity. Nucleic Acids Res. 2020;48:580–585. doi: 10.1093/nar/gkaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayomy NA, Elbakary RH, Ibrahim MA, Abdelaziz EZ. Effect of lycopene and rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Anat Rec. 2017;300:1137–1149. doi: 10.1002/ar.23525. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bera S, Greiner S, Choudhury A, et al. Dexamethasone-induced oxidative stress enhances myeloma cell radiosensitization while sparing normal bone marrow hematopoiesis. Neoplasia N Y N. 2010;12:980–992. doi: 10.1593/neo.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzović-Šarić V, Landeka I, Šarić B, et al. Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol vis. 2015;21:649–664. [PMC free article] [PubMed] [Google Scholar]
- Cervellati C, Bonaccorsi G, Cremonini E, et al. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. BioMed Res Int. 2014;2014:569563. doi: 10.1155/2014/569563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy NP, Shaver JC. Estrogens and the kidney. Kidney Int. 1974;6:366–376. doi: 10.1038/ki.1974.120. [DOI] [PubMed] [Google Scholar]
- Chung SI, Ryu SN, Kang MY (2021) Changes in bone metabolism and antioxidant defense systems in menopause-induced rats fed bran extract from dark purple rice (Oryza sativa L. Cv. Superjami). Nutrients 13(9): 2926 [DOI] [PMC free article] [PubMed]
- Consensus development conference diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- Daina A, Zoete V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Majumder R, Mandal M, Basak P. In-Silico approach for identification of effective and stable inhibitors for COVID-19 main protease (Mpro) from flavonoid based phytochemical constituents of Calendula officinalis. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1796799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz López JB, Rodríguez Rodríguez A, Ramos B, et al (2003) [Osteoporosis, estrogens, and bone metabolism. Implications for chronic renal insufficiency]. Nefrol Publicacion of Soc Espanola Nefrol 23 Suppl 2:78–83 [PubMed]
- Dissanayake KGC, Waliwita WALC, Liyanage RP. A review on medicinal uses of Zingiber officinale (ginger) Int J Health Sci and Res. 2020;10(6):142–148. [Google Scholar]
- Draper HH, Hadley M (1990) [43] Malondialdehyde determination as index of lipid Peroxidation. In: Methods in Enzymology. Academic Press, pp 421–431 [DOI] [PubMed]
- Dugasani S, Pichika MR, Nadarajah VD, et al. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol,[8]-gingerol,[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008 doi: 10.4314/ajb.v7i18.59257. [DOI] [Google Scholar]
- Ejaz AA, Mu W, Kang D-H, et al. Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol CJASN. 2007;2:16–21. doi: 10.2215/CJN.00350106. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fiebiger BJ, Erdewyk JIV, Bilal K, et al. Lipid Nephrotoxicity and increased risk of nephrolithiasis in model of estrogen deficiency. FASEB J. 2020;34:1–1. doi: 10.1096/fasebj.2020.34.s1.06026. [DOI] [Google Scholar]
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Fontella FU, Siqueira IR, Vasconcellos APS, et al. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005;30:105–111. doi: 10.1007/s11064-004-9691-6. [DOI] [PubMed] [Google Scholar]
- Gabe M (1968) Techniques histologiques
- Gabr SA, Alghadir AH, Ghoniem GA. Biological activities of ginger against cadmium-induced renal toxicity. Saudi J Biol Sci. 2019;26:382–389. doi: 10.1016/j.sjbs.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow AV, Joubert E, Hansmann CF. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chem. 1997;60:73–77. doi: 10.1016/S0308-8146(96)00312-3. [DOI] [Google Scholar]
- Ghasemzadeh A, Jaafar HZE, Rahmat A. Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Mol Basel Switz. 2010;15:7907–7922. doi: 10.3390/molecules15117907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göçer H, Gülçin İ. Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int J Food Sci Nutr. 2011;62:821–825. doi: 10.3109/09637486.2011.585963. [DOI] [PubMed] [Google Scholar]
- Gökçe A, Oktar S, Yönden Z, et al. Protective effect of caffeic acid phenethyl ester on cyclosporine A-induced nephrotoxicity in rats. Ren Fail. 2009;31:843–847. doi: 10.3109/08860220903137517. [DOI] [PubMed] [Google Scholar]
- Gong P, Chen F, Liu X, et al. Protective effect of caffeic acid phenethyl ester against cadmium-induced renal damage in mice. J Toxicol Sci. 2012;37:415–425. doi: 10.2131/jts.37.415. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. [1] Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Free radicals in biology and medicine. USA: Oxford University Press; 2015. [Google Scholar]
- Hchicha K, Korb M, Badraoui R, et al. A novel sulfate-bridged binuclear copper (II) complex: Structure, optical, ADMET and in vivo approach in a murine model of bone metastasis. New J Chem. 2021;45:13775–13784. doi: 10.1039/D1NJ02388H. [DOI] [Google Scholar]
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Zheng W, Menini S, et al. Female protection in progressive renal disease is associated with estradiol attenuation of superoxide production. Gend Med. 2007;4:56–71. doi: 10.1016/S1550-8579(07)80009-X. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kayali R, Cakatay U, Akçay T, Altuğ T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct. 2006;24:79–85. doi: 10.1002/cbf.1190. [DOI] [PubMed] [Google Scholar]
- Kim T, Ha H, Shim K-S, et al. The anti-osteoporotic effect of Yijung-tang in an ovariectomized rat model mediated by inhibition of osteoclast differentiation. J Ethnopharmacol. 2013;146:83–89. doi: 10.1016/j.jep.2012.11.037. [DOI] [PubMed] [Google Scholar]
- Köksal E, Tohma H, Kılıç Ö, et al. Assessment of antimicrobial and antioxidant activities of nepeta trachonitica: analysis of its phenolic compounds using HPLC-MS/MS. Sci Pharm. 2017 doi: 10.3390/scipharm85020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleva II, Niederländer HA, van Been TA. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal Chem. 2000;72:2323–2328. doi: 10.1021/ac9912451. [DOI] [PubMed] [Google Scholar]
- Konyalioglu S, Durmaz G, Yalcin A. The potential antioxidant effect of raloxifene treatment: a study on heart, liver and brain cortex of ovariectomized female rats. Cell Biochem Funct. 2007;25:259–266. doi: 10.1002/cbf.1328. [DOI] [PubMed] [Google Scholar]
- Lakshmi BVS, Sudhakar M. Protective effect of Zingiber officinale on gentamicin-induced nephrotoxicity in rats. IJP - Int J Pharmacol. 2010;6:58–62. doi: 10.3923/ijp.2010.58.62. [DOI] [Google Scholar]
- Li C, Qiu M, Chang L, Qi J, et al (2022) The osteoprotective role of USP26 in coordinating bone formation and resorption. Cell Death Differ: 1–14 [DOI] [PMC free article] [PubMed]
- Lin H, Decuypere E, Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 2. Short-term effect. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:745–751. doi: 10.1016/j.cbpc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mahady GB, Pendland SL, Stoia A, et al. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res PTR. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- Mangos GJ, Whitworth JA, Williamson PM, Kelly JJ. Glucocorticoids and the kidney. Nephrol Carlton Vic. 2003;8:267–273. doi: 10.1111/j.1440-1797.2003.00215.x. [DOI] [PubMed] [Google Scholar]
- Mansour SA, Mossa A-TH. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pestic Biochem Physiol. 2009;93:34–39. doi: 10.1016/j.pestbp.2008.09.004. [DOI] [Google Scholar]
- McIntosh LJ, Cortopassi KM, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: kainic acid studies. Brain Res. 1998;791:215–222. doi: 10.1016/S0006-8993(98)00104-8. [DOI] [PubMed] [Google Scholar]
- Mercantepe T, Unal D, Selli J, et al. Protective effects of estrogen and bortezomib in kidney tissue of post-menopausal rats: an ultrastructural study. Ren Fail. 2016;38:1129–1135. doi: 10.1080/0886022X.2016.1184958. [DOI] [PubMed] [Google Scholar]
- Mhadhbi N, Issaoui N, Hamadou WS, et al (2022) physico-chemical properties, pharmacokinetics, molecular docking and in-vitro pharmacological study of a cobalt (II) complex based on 2-aminopyridine.mistryselect 7(3): e202103592. 10.1002/slct.202103592
- Mishra S, Dahima R (2019) In vitro adme studies of tug-891, a gpr-120 inhibitor using swiss adme predictor. J Drug Deliv Ther 9:366–369. 10.22270/jddt.v9i2-s.2710
- Mošovská S, Nováková D, Kaliňák M. Antioxidant activity of ginger extract and identification of its active components. Acta Chim Slovaca. 2015 doi: 10.1515/acs-2015-0020. [DOI] [Google Scholar]
- Mühl H, Sandau K, Brüne B, et al. Nitric oxide donors induce apoptosis in glomerular mesangial cells, epithelial cells and endothelial cells. Eur J Pharmacol. 1996;317:137–149. doi: 10.1016/S0014-2999(96)00701-7. [DOI] [PubMed] [Google Scholar]
- Mulia MC, Wulandari CL. Literature review: the effectiveness of giving ginger (Zingiber Officinale Roscoe) to pregnant women nausea vomiting 1st trimester pregnancy. Eduvest J Univer Stud. 2021;1(8):803–809. doi: 10.36418/edv.v1i8.147. [DOI] [Google Scholar]
- Muthusami S, Ramachandran I, Muthusamy B, et al. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta Int J Clin Chem. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Mzid M, Badraoui R, Khedir SB, et al. Protective effect of ethanolic extract of Urtica urens L. against the toxicity of imidacloprid on bone remodeling in rats and antioxidant activities. Biomed Pharmacother. 2017;91:1022–1041. doi: 10.1016/j.biopha.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Nissen I, Estrada FS, Nava-Kopp AT, et al. Prolame ameliorates anxiety and spatial learning and memory impairment induced by ovariectomy in rats. Physiol Behav. 2012;106:278–284. doi: 10.1016/j.physbeh.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Ozgocmen S, Kaya H, Fadillioglu E, et al. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]
- Podkowińska A, Formanowicz D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants. 2020 doi: 10.3390/antiox9080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni F, Badraoui R, Amri N, et al. Hepatotoxicity and nephrotoxicity in rats induced by carbon tetrachloride and the protective effects of Teucrium polium and vitamin C. Toxicol Mech Method. 2019;29:313–321. doi: 10.1080/15376516.2018.1519864. [DOI] [PubMed] [Google Scholar]
- Rakshit S, Shukla P, Verma A, Kumar Nirala S, et al. Protective role of rutin against combined exposure to lipopolysaccharide and D-galactosamine-induced dysfunctions in liver, kidney, and brain: Hematological, biochemical, and histological evidences. J Food Biochem. 2021;45(2):e13605. doi: 10.1111/jfbc.13605. [DOI] [PubMed] [Google Scholar]
- Rashedinia M, Khoshnoud MJ, Fahlyan BK, et al (2020) Syringic acid: A potential natural compound for the management of renal oxidative stress and mitochondrial biogenesis in diabetic rats. Curr Drug Discov Technol [DOI] [PubMed]
- Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, Sassa S, Kudo H, et al. Preventive effects of a herbal medicine on bone loss in rats treated with a GnRH agonist. Eur J Endocrinol. 2000;143:139. doi: 10.1530/eje.0.1430139. [DOI] [PubMed] [Google Scholar]
- Sanjeev S, Bidanchi RM, Murthy MK, et al. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res. 2019;26:20631–20653. doi: 10.1007/s11356-019-05420-7. [DOI] [PubMed] [Google Scholar]
- Sanz AB, Sanchez-Niño MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80:708–718. doi: 10.1038/ki.2011.180. [DOI] [PubMed] [Google Scholar]
- Sassa S, Sakamoto S, Zhou YF, et al. Preventive effects of a Chinese herbal medicine, hochu-ekki-to, on bone loss in ovariectomized rats. Vivo Athens Greece. 2001;15:25–28. [PubMed] [Google Scholar]
- Sayakhot P, Vincent A, Deeks A, Teede H. Potential adverse impact of ovariectomy on physical and psychological function of younger women with breast cancer. Menopause N Y N. 2011;18:786–793. doi: 10.1097/gme.0b013e318204af9d. [DOI] [PubMed] [Google Scholar]
- Scarano A, Ceccarelli M, Marchetti M, Piattelli A, et al. Soft tissue augmentation with autologous platelet gel and β-TCP: a histologic and histometric study in mice. BioMed Res Int. 2016;2016:2078104. doi: 10.1155/2016/2078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selli J, Unal D, Mercantepe F, et al. Protective effects of beta glucan in brain tissues of post-menopausal rats: a histochemical and ultra-structural study. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2016;32:234–239. doi: 10.3109/09513590.2015.1110139. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Singh M. Cancer preventive properties of ginger: A brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Smets P, Meyer E, Maddens B, Daminet S. Cushing’s syndrome, glucocorticoids and the kidney. Gen Comp Endocrinol. 2010;169:1–10. doi: 10.1016/j.ygcen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Stoilova I, Krastanov A, Stoyanova A, et al. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. doi: 10.1016/j.foodchem.2006.06.023. [DOI] [Google Scholar]
- Sverrisson K, Axelsson J, Rippe A, et al. Acute reactive oxygen species (ROS)-dependent effects of IL-1β, TNF-α, and IL-6 on the glomerular filtration barrier (GFB) in vivo. Am J Physiol-Ren Physiol. 2015;309:F800–F806. doi: 10.1152/ajprenal.00111.2015. [DOI] [PubMed] [Google Scholar]
- Tang J, Yan H, Zhuang S. Inflammation and oxidative stress in obesity-related glomerulopathy. Int J Nephrol. 2012;2012:e608397. doi: 10.1155/2012/608397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. Lead finding from Phyllanthus debelis with hepatoprotective potentials. Asian Pac J Trop Biomed. 2012;2:S1735–S1737. doi: 10.1016/S2221-1691(12)60486-9. [DOI] [Google Scholar]
- Vipin AV, Rao R, Kurrey NK, et al. Protective effects of phenolics rich extract of ginger against Aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed Pharmacother. 2017;91:415–424. doi: 10.1016/j.biopha.2017.04.107. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Gausson V, Nguyen A-T, et al. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64:82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Liu C, Jiang J, et al. Ginger extract diminishes chronic fructose consumption-induced kidney injury through suppression of renal overexpression of proinflammatory cytokines in rats. BMC Complem Altern Med. 2014;14:174. doi: 10.1186/1472-6882-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yc M. A bioavailability score. J Med Chem. 2005 doi: 10.1021/jm0492002. [DOI] [PubMed] [Google Scholar]
- You J-M, Yun S-J, Nam KN, et al. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can J Physiol Pharmacol. 2009;87:440–447. doi: 10.1139/y09-027. [DOI] [PubMed] [Google Scholar]
- Zahedi A, Nematbakhsh M, Moeini M, Talebi A (2015) Role of endothelin receptor antagonist; bosentan in cisplatin-induced nephrotoxicity in ovariectomized estradiol treated rats. J Nephropathol 4:134–140. 10.12860/jnp.2015.25 [DOI] [PMC free article] [PubMed]
- Zammel N, Amri N, Chaabane R, et al. Proficiencies of Zingiber officinale against spine curve and vertebral damage induced by corticosteroid therapy associated with gonadal hormone deficiency in a rat model of osteoporosis. Biomed Pharmacother. 2018;103:1429–1435. doi: 10.1016/j.biopha.2018.04.159. [DOI] [PubMed] [Google Scholar]
- Zammel N, Oudadesse H, Allagui I, et al. Evaluation of lumbar vertebrae mineral composition in rat model of severe osteopenia: a Fourier Transform Infrared Spectroscopy (FTIR) analysis. Vibrat Spectrosc. 2021;115:103279. doi: 10.1016/j.vibspec.2021.103279. [DOI] [Google Scholar]
- Zammel N, Saeed M, Bouali N, et al. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum. In Silico, Biochemical and Histological Study. Foods. 2021;10:1383. doi: 10.3390/foods10061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Pippin JW, Krofft RD, et al. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol-Ren Physiol. 2013;304:F1375–F1389. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. Research on antioxidant activity of flavonoids from natural materials. Food Chem. 1999;64:e9. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]