Figure 8.
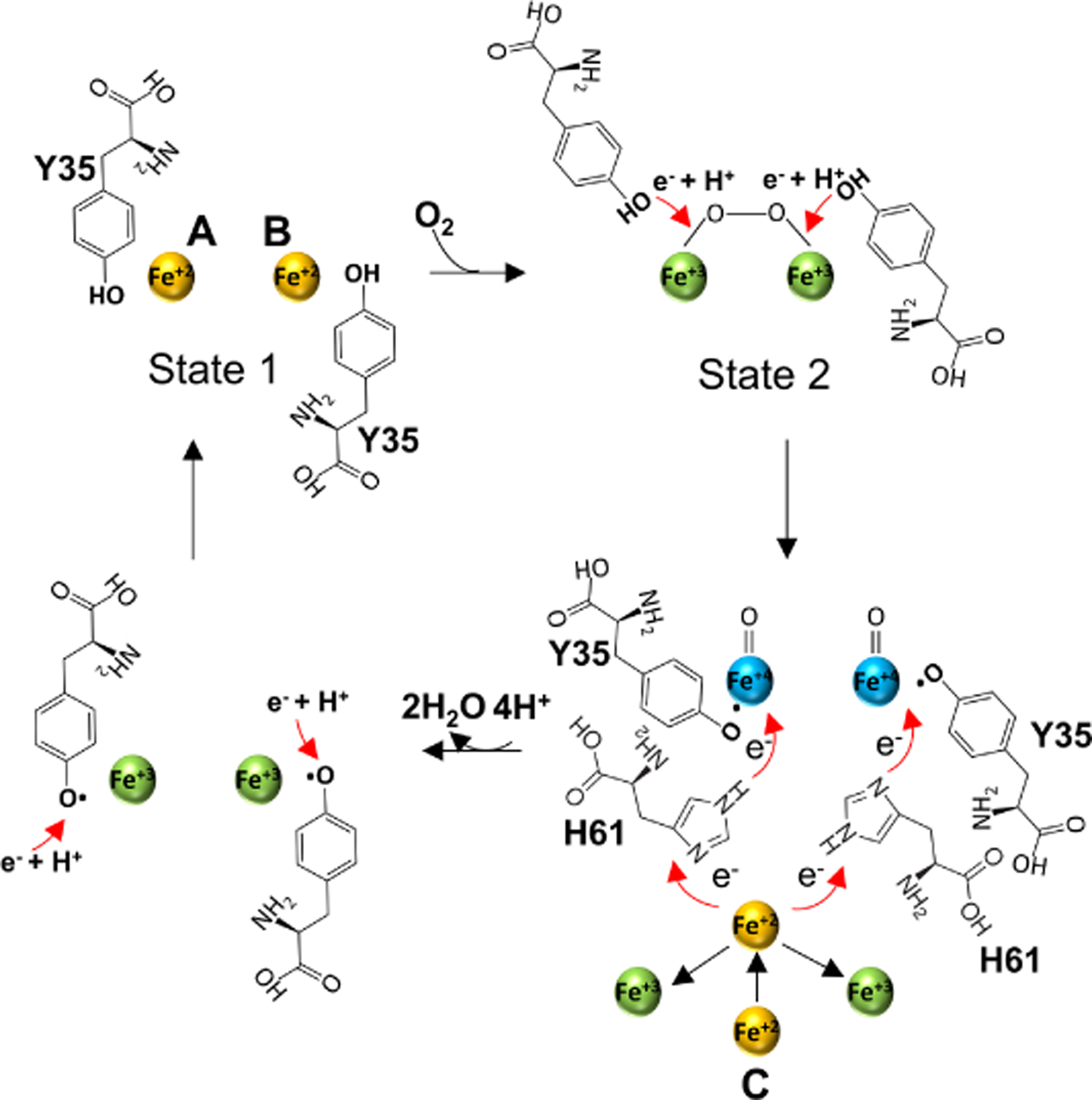
Proposed mechanism of Fe+2 oxidation at the FOC of EncB. In step 1, the 2 Fe+2 atoms in the “high-affinity” binding state (state 1) interact with O2 to form a peroxodiferric intermediate. In step 2, the two conserved Y35 move towards the peroxodiferric intermediate to donate e− + H+ to the Fe+2 atoms forming two tyrosyl radicals and two hypothetical Fe+4 intermediates with an unknown oxidation state. In step 3, one of the two Fe+4 is reduced to Fe+3 through an electro-transfer chain formed by the site C Fe+2 and H61 releasing a H2O molecule. The oxidized site C Fe+3 leaves the site and is replaced by a second Fe+2 which in turn reduces the second Fe+4 releasing a second H2O. In step 4 the tyrosyl radical is reduced by an unidentified redox partner followed by the addition of a proton. Finally, Fe+3 atoms at the FOC are displaced by the incoming Fe+2. See also Figure S10.
