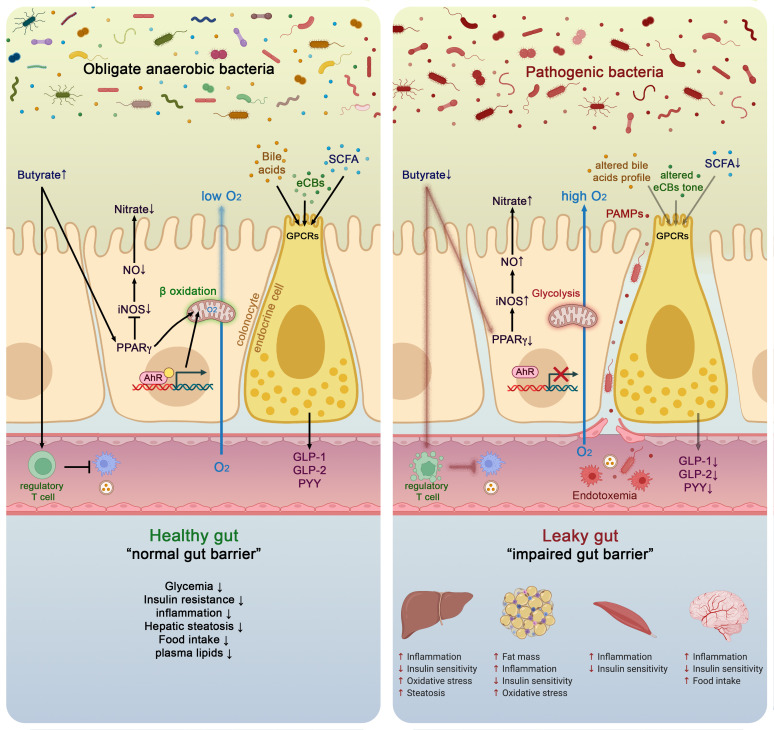
Figure 3.
Molecular mechanisms linking gut microbiota and host health in both healthy and pathological situation. In healthy situation, colonocytes use butyrate as energy substrate via the beta-oxidation in the mitochondria, thereby consuming oxygen and directly contributing to maintain anaerobic condition in the lumen. Butyrate also binds to peroxisome proliferator-activated receptor gamma (PPARγ) which in turn repress inducible nitric oxide synthase (iNOS), decreases nitric oxide production (NO) and eventually nitrate production. Conversely, in pathological situations low butyrate content in the lumen is associated with lower PPARγ activity, increased glycolysis and lower oxygen consumption. This is associated with a higher expression of iNOS which in turn produces more NO and eventually increases nitrates availability for specific pathogens. Butyrate can also stimulate immune cells such as regulatory T cells (Treg) to reduce inflammation. The nuclear transcription factor aryl hydrocarbon receptor (AhR) is highly expressed and activated in healthy colonocytes, whereas agonists of AhR are lower or reduced AhR activity can lead to altered gut barrier function. Enteroendocrine cells (L-cells) are expressing several key receptors activated by short chain fatty acids (SCFAs), specific endocannabinoids (eCBs) and bile acids (BAs). Activating these receptors increase the secretion of key gut peptides such as glucagon-like peptide (GLP)-1, GLP-2 and peptide YY (PYY). Altogether, the interaction between the gut microbes and these molecular actors contributes to reduce intestinal permeability, to improve insulin secretion and insulin sensitivity, to reduce food intake, to lower plasma lipids and to avoid hepatic steatosis and metabolic endotoxaemia. All these effects are associated with lower inflammation. Conversely, opposite effects have been observed in pathological situations.