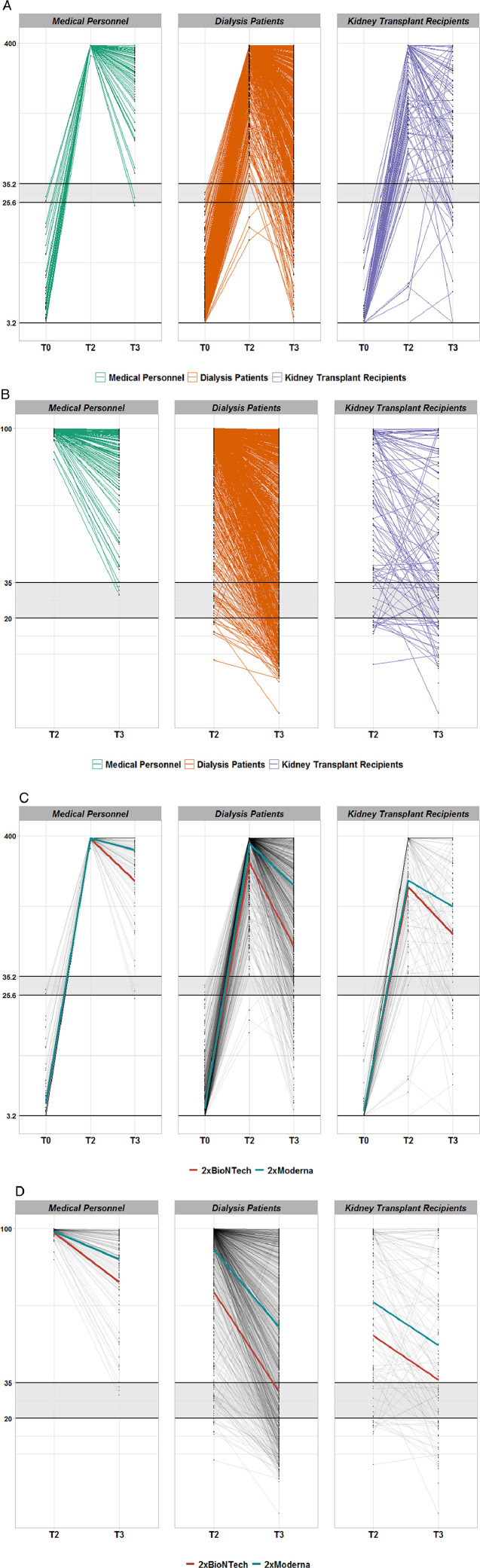
Figure 2.
Time course of anti-SARS-CoV-2 IgG (A/C) or receptor binding domain (B/D) antibodies in seroconverted Dia-Vacc study participants. Figure 2A: IgG against S1 protein in different study groups. Each thin line corresponds the anti-Spike S1 protein IgG antibody values (QuantiVac, Euroimmun) of a study participant from T0 (vaccination start) via T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. All three patient groups are represented (MP-green, DP-red, KTR-blue). The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit BAU/ml. Figure 2B: Receptor binding domain antibodies against S1 protein in different study groups. Each thin line corresponds the anti-Spike S1 protein RBD-IgG antibody values (Euroimmun) of a study participant from T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. All three patient groups are represented (MP-green, DP-red, KTR-blue). The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit % inhibition. Figure 2C: IgG against S1 protein dependent on vaccine type. Each thin line corresponds the anti-Spike S1 protein IgG antibody values (QuantiVac, Euroimmun) of a study participant from T0 (vaccination start) via T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). The thicker lines represent average responses by vaccine type (red-BNT162b2mRNA and blue 1273-mRNA) in each group. Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit BAU/ml. Figure 2D: Receptor binding domain antibody against S1 protein dependent on vaccine type. Each thin line corresponds the anti-Spike S1 protein RBD-IgG antibody values (Euroimmun) of a study participant from T0 (vaccination start) via T2 (eight weeks after vaccination start) to T3 (six months after vaccination start). The thicker lines represent average responses by vaccine type (red-BNT162b2mRNA and blue 1273-mRNA) in each group. Only patients with successful de novo seroconversion at T2 (IgA or IgG antibody positivity against the SARS-CoV-2 S1 protein) after 2x mRNA vaccination and without SARS-CoV-2 nucleocapsid (NCP) antibodies were considered. The area shaded grey designates IgG range below positivity level. The vertical axis is depicted on scale with corresponding unit % inhibition.