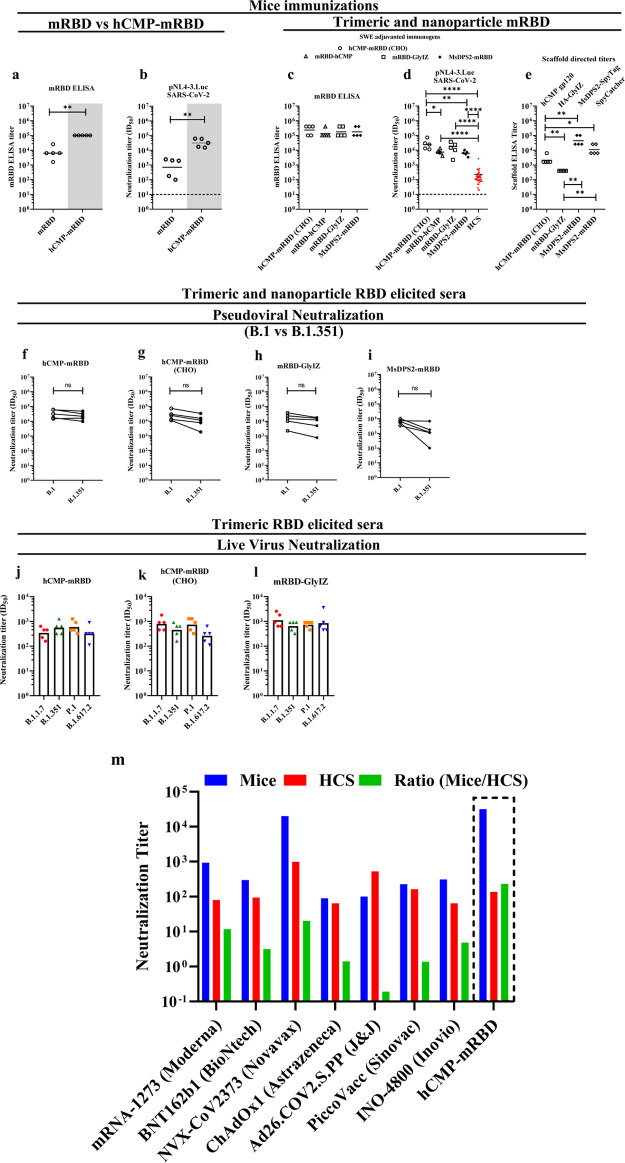
Figure 3.
ELISA and pseudovirus neutralization with sera elicited at weeks 0, 3 after two immunizations with SWE adjuvant-containing formulations. (a,b) Immunization with mRBD (white panel) or hCMP-mRBD (gray panel) (n = 5 mice/group). (c–e) Immunizations with mRBD-hCMP, mRBD-GlyIZ, or MsDPS2 nanoparticle displaying mRBD (n = 5 mice/group). Pseudoviral neutralization titers utilized pNL4–3.Luc. SARS-CoV-2 D614G Δ19. HCS: Human Convalescent Sera (n = 40). (e) ELISA binding titer against scaffolds hCMP, GlyIZ trimerization domain, MsDPS2 SpyTag, and SpyCatcher. (f–i) Pseudoviral neutralization titers against wildtype and pseudovirus with B.1.351 RBD mutations. The paired comparisons were performed utilizing the Wilcoxon Rank-Sum test in f–i. The black solid horizontal lines in each scatter plot represent Geometric Mean Titer (GMT). The pairwise titer comparisons were performed utilizing two-tailed Mann–Whitney tests in a–e (* indicates P < 0.05, ** indicates P < 0.01, **** indicates P < 0.0001). (j–l) Live virus neutralization against B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.671.2 (Delta). The paired comparisons were performed utilizing ANOVA in j–l, and no significant differences were seen. The histograms in each plot represent geometric mean titer (GMT). (m) Neutralizing antibody titers in mice (blue), in human convalescent sera (HCS) (red) assayed in the identical assay platform, and their relative ratio (green). Values for a number of vaccine candidates being tested in the clinic or provided with emergency use authorizations are shown58−71 and corresponding values for hCMP-RBD are boxed.