Abstract
Rationale
Limitation of physical activity is a common presenting complaint for patients with pulmonary arterial hypertension (PAH). Physical activity is thought to be determined by cardiopulmonary function, yet there are limited data that investigate this relationship.
Objectives
We aimed to study the relationship between right ventricular function and daily activity and its impact on health-related quality of life (HRQoL) in PAH.
Methods
Baseline data for 55 patients enrolled in PHANTOM (Pulmonary Hypertension and Anastrozole), an ongoing multicenter randomized controlled trial of anastrozole in PAH, were used. Postmenopausal women and men were eligible and underwent 6-minute walk testing and echocardiography and completed HRQoL questionnaires. Each patient wore an accelerometer for 7 days. Multivariable linear regression models were used to study the association between tricuspid annular plane systolic excursion (TAPSE) and vector magnitude counts, and between daily activity and HRQoL. Principal component analysis and K-means clustering were used to identify activity-based phenotypes. K-nearest neighbors classification was applied to an independent cross-sectional cohort from the University of Pennsylvania.
Results
The mean age of patients in PHANTOM was 61 years. In total, 67% were women with idiopathic PAH as the most common etiology. A 0.4-cm increase in TAPSE was associated with an increase in daily vector magnitude counts (β: 34,000; 95% confidence interval [CI], 900–67,000; P = 0.004) after adjustment for age, sex, body mass index, etiology of PAH, and wear time. A 1-SD increase in vector magnitude counts was associated with higher 6-minute walk distance (β: 56.1 m; 95% CI, 28.6–83.7; P < 0.001) and lower emPHasis-10 scores (β: −3.3; 95% CI, 0.3–6.4; P = 0.03). Three activity phenotypes, low, medium, and high, were identified. The most active phenotype had greater 6-minute walk distances (P = 0.001) and lower emPHasis-10 scores (P = 0.009) after adjustment for age, sex, body mass index, World Health Organization functional class, and parenteral prostacyclin use. Phenotypes of physical activity were reproduced in the second cohort and were independently associated with 6-minute walk distance.
Conclusions
Better right ventricular systolic function was associated with increased levels of activity in PAH. Increased daily activity was associated with greater 6-minute walk distance and better HRQoL. Distinct activity-based phenotypes may be helpful in risk stratification of patients with PAH or provide novel endpoints for clinical trials.
Keywords: pulmonary hypertension, physical activity, quality of life, accelerometry
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by increased pulmonary vascular resistance, increased pulmonary arterial pressures, and right ventricular (RV) dysfunction (1). Limitation in physical activity is one of the earliest manifestations of PAH. Physical activity is any body movement that requires more energy than resting (2, 3) and is affected by cardiac, respiratory, and musculoskeletal function, as well as emotional, social, and environmental factors. These different domains collectively determine behaviors and attitudes that impact the patient experience of activity (4, 5). Although the terms are often used interchangeably, physical activity encompasses more than just exercise, a subset of physical activity that is planned, structured, and repetitive and has as a final or an intermediate objective to improve or maintain physical fitness (3).
Intuitively, we expect that worse underlying PAH leads to reduced daily activity. However, data to support this conventional thinking are limited. Prior studies have found that patients with PAH are more sedentary and have reduced health-related quality of life (HRQoL) compared with age- and sex-matched controls (6–9). A small single-center study showed that step counts in this population correlated with 6-minute walk distance and generic measures of HRQoL (such as the European quality of life index) (10). However, the role of RV function in determining physical activity and the association of daily physical activity with 6-minute walk distance and PAH-specific HRQoL are poorly understood.
PAH care has traditionally focused on exercise capacity as a marker of disease progression and as an endpoint in clinical trials (1, 11–13). This has been assessed clinically using standardized tests such as the 6-minute walk test (14). By design, this test can currently only be performed episodically in a clinical setting (15) and has unclear pertinence to day-to-day limitations. Alternatively, triaxial accelerometers are easy-to-use devices that provide valid, objective, and continuous measures of physical activity throughout the day in the patient’s home environment (16–18). To our knowledge, no prior studies have investigated the associations of objectively measured physical activity with disease severity in patients with PAH receiving care across multiple centers in the United States.
In this study, our goal was to understand the role of physical activity in PAH. Specifically, we aimed to assess the relationship of RV systolic function, quantified by the tricuspid annular plane systolic excursion (TAPSE), with physical activity measured using accelerometry. TAPSE measures the systolic displacement of the tricuspid valve annulus toward the RV apex and is simple to perform and reproducible. It has been studied extensively in PAH as a reliable measure of RV systolic function and has prognostic significance with lower values indicating worse RV function (1, 19, 20). We hypothesized that patients with PAH who have better RV function (higher TAPSE) have higher overall physical activity and that the patients with higher activity would have better HRQoL. Additionally, we sought to determine if we could phenotype patients with PAH based on physical activity patterns and reproduce the phenotypes in an independent cohort of patients with PAH.
Methods
Study Samples
We performed a cross-sectional analysis of the baseline (prerandomization) data from the PHANTOM (Pulmonary Hypertension and Anastrozole) trial, an ongoing NHLBI-funded multicenter placebo-controlled trial (NCT03229499). This trial enrolled adult men and postmenopausal women with PAH on stable therapy at the University of Pennsylvania, Stanford University, University of Colorado Denver, Johns Hopkins University, Washington University, Rhode Island Hospital, and Vanderbilt University. The study excluded patients who were hospitalized, acutely ill, with World Health Organization (WHO) functional class IV, or those limited by musculoskeletal function or coordination. Patients with contraindications to anastrozole such as osteoporosis, ongoing treatment with hormone replacement therapy, and a history of breast cancer were excluded (see Table E1 in the online supplement). Prerandomization data from the first 55 patients enrolled in the trial between 2018 and 2020 before the coronavirus disease (COVID-19) pandemic were included.
For the validation analysis, we utilized an independent cross-sectional single-center cohort of 60 women with PAH at the University of Pennsylvania enrolled between 2015 and 2017. Premenopausal women were included in this study, but inclusion and exclusion criteria were otherwise similar to those of PHANTOM. No subjects were in both studies. Both studies were approved by the institutional review board, and all subjects provided informed consent.
Data Collection
In both cohorts, subjects attended a study visit, during which demographics and medical histories were recorded. Subjects performed 6-minute walk testing using standardized methods (15) and underwent protocolized research grade echocardiograms. All echocardiograms were interpreted centrally at the Mayo Echo Core Laboratory (PHANTOM) and the Penn Echo Core (Penn cohort) according to the Society of Echocardiography guidelines from 2015 (21). Echocardiography readers were blinded to other clinical information. TAPSE was chosen a priori as the primary variable of interest as described above, especially given challenges with transthoracic and subcostal windows on echocardiography in the setting of RV dilatation. Other parameters of RV systolic function, including RV fractional area change and Tei index, were also assessed as secondary variables of interest (22).
Patients completed the Medical Outcome Study Short Form-36 (SF-36) and the emPHasis-10 (E-10) during their study visit. SF-36 is designed to assess general HRQoL across two domains, mental and physical (23). The mental component score was calculated utilizing the vitality, social functioning role, emotional, and mental health domains. The physical component score was calculated using the physical functioning role, physical pain, and general health perception domains (23). The E-10 is a pulmonary hypertension (PH) specific instrument that consists of questions addressing breathlessness, fatigue, confidence, and control. Each item was scored on a six-point scale from 0 to 5, and the total score ranges from 0 to 50 with higher scores indicating worse HRQoL with a reliability coefficient of 0.95 (24, 25). The SF-36 and E-10 have been validated and widely used in PAH (24, 26–29).
Activity Measures
In PHANTOM, all patients were instructed to wear the ActiGraph GT9X accelerometer on the nondominant hip during waking hours for a 7-day period after the baseline study visit and before starting study drug. In the Penn cohort, patients were instructed to wear the ActiGraph GT3X monitor on their nondominant wrist for 24 hours for a 7-day period. Nonwear time was defined using a standardized algorithm (16, 30, 31). Days with less than 5 hours of wear time were excluded in both cohorts (32). Patients removed the ActiGraphs for water-based activities such as bathing.
These accelerometers gather data along three orthogonal axes: anteroposterior, vertical, and mediolateral at a frequency of 30 Hz. The data were processed using the ActiLife software into epoch lengths of 1 minute and summarized as vector magnitude counts that reflect the mean amplitude deviation across the three axes, calculated by obtaining the square root of the quadrate of the three separate dimensional axes [(x2 + y2 + z2)1/2] (16). We used the total daily vector magnitude counts as the primary output feature from accelerometers. Secondary output features included step counts and time spent in sedentary activity (1.0–1.5 METs), light activity (1.6–3.0 METs), moderate activity (3.1–6.0 METs), or vigorous activity (>6.1 metabolic equivalent of tasks [METs]) (33). In this study, we expressed the accelerometer data as wear time in minutes, vector magnitude counts, step counts, and minutes spent in different activity intensities. The median value of activity metrics summed over wear time was calculated as robust measures of activity intensities for each subject and was used in the analyses.
In both cohorts, patients maintained an activity diary (Table E2), where they recorded times when they were asleep and when they performed intentional exercise. Activity diaries were used as a quality control measure to evaluate algorithm-generated ActiGraph wear times.
Statistical Analysis
Data were summarized using means and SDs or medians and interquartile ranges, as appropriate. Heatmaps were used to visualize physical activity over the 7-day period. We conducted two sets of regression analyses to evaluate the associations between echocardiographic functioning and daily physical activity and physical activity with patients’ self-reported outcomes, respectively. First, a linear regression model was used to estimate the association between TAPSE (independent variable) and vector magnitude counts (dependent variable). Other independent and dependent variables included qualitative RV dysfunction (as a categorical variable), RV fractional area change, Tei index, and step counts, respectively.
We then used separate models to assess the association between vector magnitude counts (independent variable) and 6-minute walk distance and E-10 and SF-36 scores (dependent variables). All models were adjusted a priori for age, sex, body mass index, PAH attributable to connective tissue disease versus other types, and accelerometer wear time.
To determine if there were unique activity phenotypes in PHANTOM, we utilized median values of seven accelerometer features, including wear time, vector magnitude counts, step counts, and minutes spent in sedentary, light, moderate and vigorous activity for each patient (7 features × 55 subjects = 385 data points). Features were standardized by mean centering and SD scaling for harmonization before performing a principal component (PC) analysis (34–36). Accelerometer wear time was included in the PC analysis, as it was highly correlated with other measures. K-means clustering was then performed on the PC scores, which identified three nonoverlapping activity phenotypes (34, 37, 38). Elbow and silhouette methods were used to identify the optimal number of clusters to partition the data set (34). Summary statistics were obtained for patients within each cluster. After cluster labels were assigned, we conducted post hoc analyses to compare characteristics across three clusters. Multivariable linear regression models were then used to assess the association of underlying TAPSE with cluster assignment. Additionally, multivariable linear regression models were performed to estimate the difference in 6-minute walk distance and HRQoL across the three clusters. Based on a priori hypotheses and differences in clinical parameters noted among the three clusters, models were adjusted for age, sex, body mass index, WHO functional class, and use of parenteral prostacyclin analogs. Models were selected using lowest Akaike’s information criteria (AIC) and Bayesian Information criteria (BIC) values. Additional covariates assessed included oxygen use and PAH attributable to connective tissue disease versus other types.
To examine the reproducibility of clustering in PHANTOM data, we projected similar key accelerometer features collected in the Penn cohort and centered and scaled these data. These features from the Penn cohort were then projected onto the PCs generated from PHANTOM. A K-nearest neighbors analysis was performed to segregate patients into nonoverlapping activity-based clusters (34, 39, 40). Two subjects had ties for cluster assignment and were excluded from further analysis. A statistical modeling approach similar to those outlined above were performed to determine if associations in the PHANTOM cohort were also seen in the Penn cohort. All statistical analyses were performed in R 3.6.2 (R Foundation for Statistical Computing).
Results
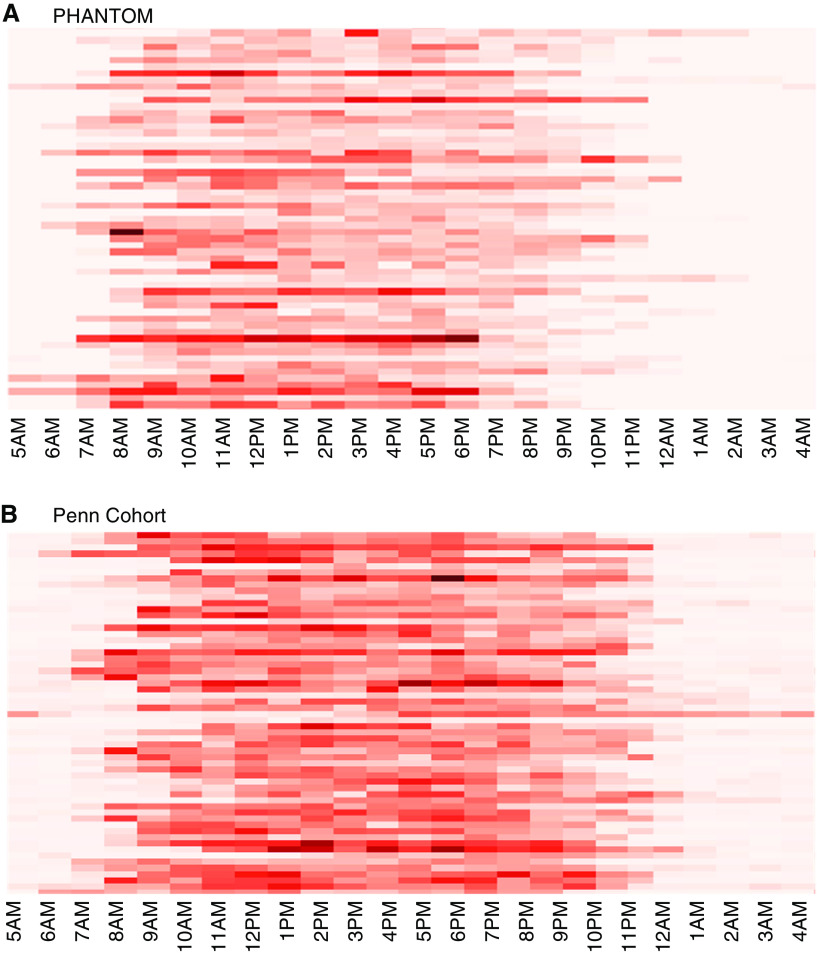
The PHANTOM cohort included 55 subjects. The mean age was 61 years, and 67% were women (Table 1). Idiopathic PAH was the most common etiology, and 22% patients were receiving parenteral prostacyclin analogs. Patients wore the ActiGraph an average of 13.6 hours each day. Wear times varied greatly among patients. (Figure E1A). Four patients had days with fewer than 5 hours of wear time, two patients with 1 day each and two patients with 2 days each, excluded from analyses. Most patients were WHO functional class II. Patients spent the vast majority of their time being sedentary and a small amount performing light activity. No vigorous activity was observed in this cohort. Over the week that the ActiGraph was worn, there was significant variability in activity between patients depending on time of the day (Figure 1A).
Table 1.
Patient characteristics (PHANTOM)
| n = 55 | |
|---|---|
| Age, yr | 61 ± 10.3 |
| Sex, female, % | 67 |
| Body mass index, kg/m2 | 30.1 ± 6.4 |
| Race, % | |
| White | 80 |
| African American | 7 |
| Other | 13 |
| PAH etiology, % | |
| Idiopathic/heritable | 49 |
| Connective tissue disease | 24 |
| Other | 27 |
| PAH medications, % | |
| Only oral | 67 |
| Inhaled ± oral | 11 |
| Parenteral prostacyclin analog ± oral | 22 |
| WHO functional class, % | |
| I | 7 |
| II | 64 |
| III | 29 |
| emPHasis-10 | 19 ± 9 |
| SF-36–MCS | 55 ± 7 |
| SF-36–PCS | 37 ± 8 |
| 6-minute walk distance, m | 424 ± 113 |
| TAPSE, cm | 2.2 (1.9–2.6) |
| Right ventricular dysfunction, % | |
| None | 11 |
| Mild | 42 |
| Moderate | 36 |
| Severe | 11 |
| Accelerometry variables (per day) | |
| Vector magnitude counts | 355,600 ± 158,400 |
| Step counts | 3,860 ± 2,830 |
| Sedentary, min | 610 (508–680) |
| Light activity, min | 165 (131–190) |
| Moderate activity, min | 8 (2–13) |
Definition of abbreviations: MCS = mental component score; PAH = pulmonary arterial hypertension; PCS = physical component score; PHANTOM = Pulmonary Hypertension and Anastrozole; SF-36 = Short form-36; TAPSE = tricuspid annular plane systolic excursion; WHO = World Health Organization.
Data are summarized as mean ± SD or median (interquartile range).
Figure 1.
Vector magnitude counts over a 7-day period from (A) PHANTOM (Pulmonary Hypertension and Anastrozole) and (B) Penn (Pennsylvania) cohort. These heatmaps show vector magnitude counts for subjects across the 7-day study period. Each row represents data from an individual patient. Each graphical square depicts the median vector magnitude counts for that hour over the 7-day study period.
Association of RV Function with Daily Physical Activity
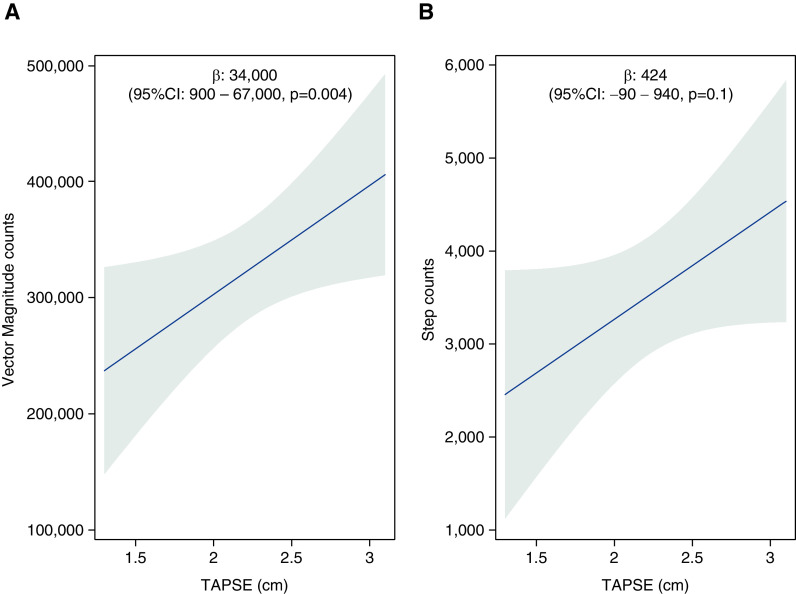
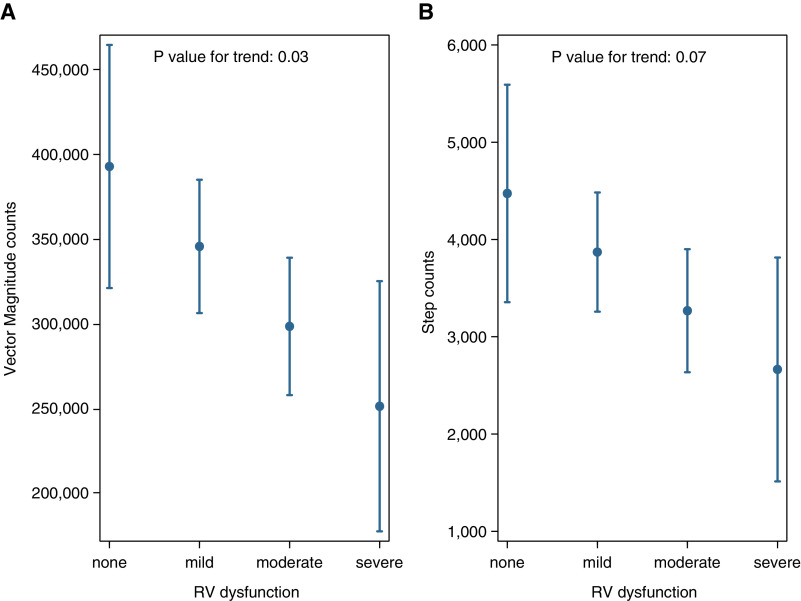
We found that a higher TAPSE (better RV function) was significantly associated with higher vector magnitude counts in the PHANTOM cohort. A 0.4-cm or 1-SD increase in TAPSE was associated with an increase in vector magnitude counts by 34,000 (95% CI, 900 to 67,000; P = 0.004) and possibly an increase in step counts by 424 steps (95% CI, −90 to 940 steps; P = 0.10) after adjustment for covariates (Figures 2A and 2B). Similarly, patients with more severe qualitative RV dysfunction had significantly lower vector magnitude counts and a trend toward lower step counts (Figures 3A and 3B). Analyses including other RV systolic function measures (RV fractional area change and Tei Index) were not statistically significant, but patients with higher RV fractional area change and lower Tei Index (better RV systolic function) had quantitatively higher levels of activity (Table E3).
Figure 2.
Multivariable linear regression models of tricuspid annular plane systolic excursion (TAPSE) with (A) vector magnitude counts and (B) step counts after adjusting for age, sex, body mass index, connective tissue disease as etiology of pulmonary arterial hypertension and accelerometer wear time in PHANTOM (Pulmonary Hypertension and Anastrozole). (β coefficients reported per 0.4-cm increase in TAPSE. Gray area represents the 95% confidence interval [CI].)
Figure 3.
Expected mean estimates for (A) vector magnitude counts and (B) step counts by right ventricular dysfunction based on regression models after adjusting for age, sex, body mass index, connective tissue disease as etiology of pulmonary arterial hypertension, and accelerometer wear time in PHANTOM (Pulmonary Hypertension and Anastrozole). RV = right ventricular.
Association of Daily Physical Activity with Outcomes
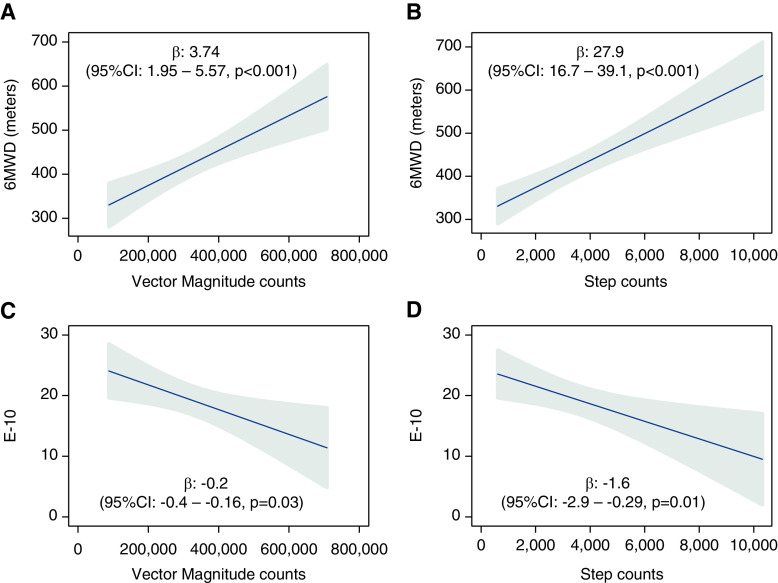
A higher level of daily physical activity, measured by vector magnitude counts, was associated with a higher 6-minute walk distance and lower E-10 scores, indicative of better HRQoL in the PHANTOM cohort (Figure 4). A 1-SD increase in vector magnitude counts was associated with a 56.1-m increase in 6-minute walk distance (95% CI, 28.6–83.7 m; P < 0.001) and 3.3-point decrease in E-10 scores (95% CI, 0.3–6.4; P = 0.03). Similar associations were found for step counts.
Figure 4.
Multivariable linear regression models of 6-minute walk distance with (A) vector magnitude counts and (B) step counts and emPHasis-10 scores with (C) vector magnitude counts and (D) step counts after adjusting for age, sex, body mass index, connective tissue disease as etiology of pulmonary arterial hypertension, and accelerometer wear time in PHANTOM (Pulmonary Hypertension and Anastrozole). (β coefficients reported for a 10,000 increase in vector magnitude counts and 1,000 increase in step counts; gray area represents the 95% confidence interval [CI].) 6MWD = 6-minute walk distance; E-10 = emPHasis-10.
Physical Activity Phenotypes
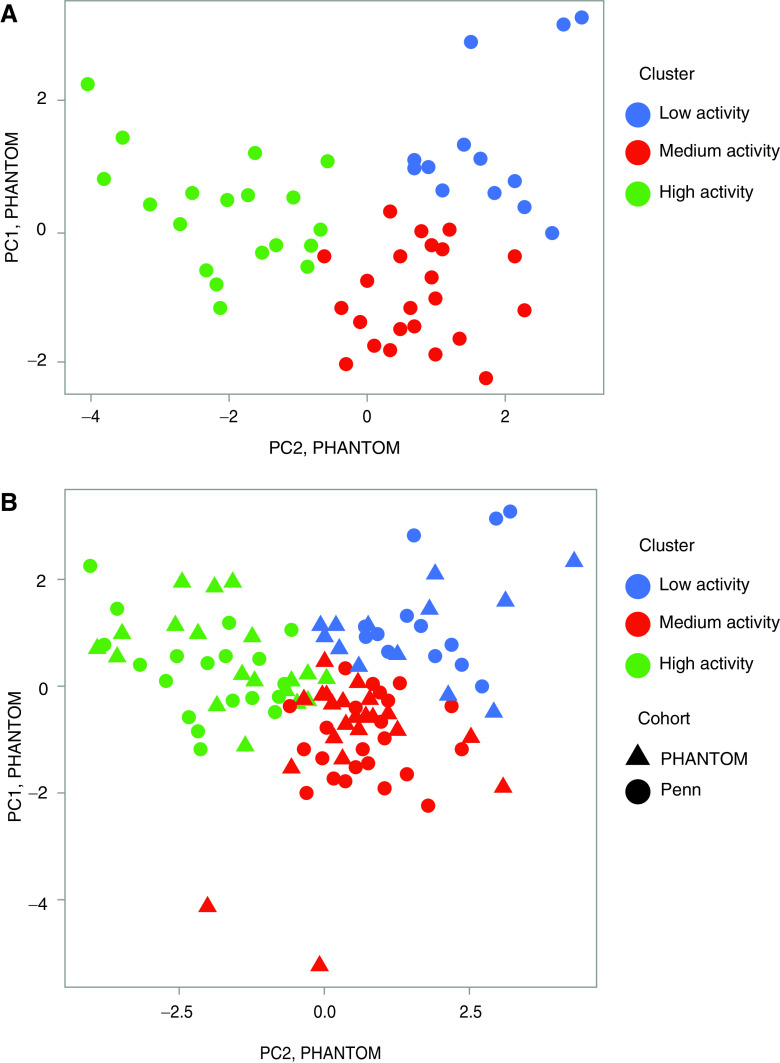
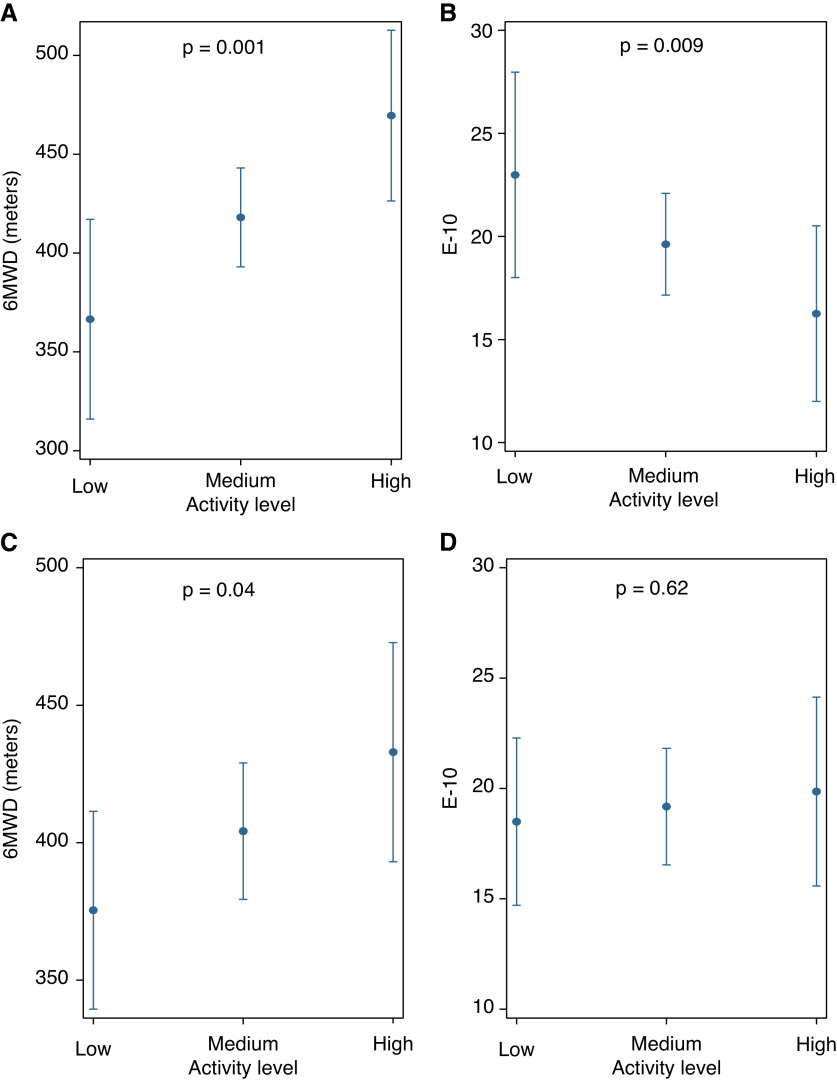
We identified three distinct nonoverlapping clusters of subjects in PHANTOM using PC analysis and K-means clustering, those with low activity, medium activity, and high activity (Figure 5A and Table E4). The most active cluster was younger and more likely to be male and to have idiopathic or heritable PAH (Table 2). Less active patients had worse WHO functional class. Across the three clusters (from the lowest activity to highest activity), 6-minute walk distance significantly increased, and E-10 scores were significantly lower (indicating better HRQoL) even after adjustment for age, sex, BMI, WHO functional class, and use of parenteral prostacyclin analogs (Figures 6A and 6B). No association was found between TAPSE and cluster assignment or cluster assignment and components of the SF-36 score (Table E5).
Figure 5.
(A) K-means clustering (PHANTOM [Pulmonary Hypertension and Anastrozole]); (B) K-nearest neighbors clustering (Penn Cohort). PC = principal component.
Table 2.
Patient characteristics across activity clusters
| Activity Level | PHANTOM |
Penn Cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Low (n = 13) | Medium (n = 23) | High (n = 19) | P Value | Low (n = 25) | Medium (n = 13) | High (n = 19) | P Value | |
| Age, yr | 66 ± 7 | 63 ± 9 | 54 ± 11 | <0.001 | 48 ± 16 | 52 ± 23 | 48 ± 16 | 0.77 |
| Sex, female, % | 85 | 74 | 58 | 0.24 | — | — | — | — |
| Body mass index, kg/m2 | 29 ± 6 | 31 ± 7 | 29 ± 5 | 0.42 | 28 ± 6 | 27.5 ± 8 | 27.2 ± 4 | 0.89 |
| PAH etiology, % | 0.09 | 0.73 | ||||||
| Idiopathic/heritable | 15 | 57 | 63 | — | 48 | 38 | 58 | — |
| Connective tissue disease | 39 | 22 | 16 | — | 28 | 39 | 32 | — |
| Other | 46 | 21 | 21 | — | 24 | 23 | 10 | — |
| PAH medications, % | 0.17 | 0.07 | ||||||
| Only oral | 69 | 65 | 68 | — | 48 | 62 | 63 | — |
| Inhaled ± oral | 8 | 22 | 0 | — | 4 | 30 | 0 | — |
| Parenteral prostacyclin analog ± oral | 23 | 13 | 32 | — | 48 | 8 | 37 | — |
| WHO functional class, % | 0.008 | 0.26 | ||||||
| I | 0 | 0 | 21 | — | 0 | 15 | 16 | — |
| II | 54 | 61 | 74 | — | 48 | 54 | 37 | — |
| III | 46 | 39 | 5 | — | 52 | 31 | 47 | — |
Definition of abbreviations: PAH = pulmonary arterial hypertension; Penn = Pennsylvania; PHANTOM = Pulmonary Hypertension and Anastrozole; WHO = World Health Organization.
Low, medium, and high indicate activity levels. Data are summarized as n % or median (interquartile range).
Figure 6.
(A–D) Expected mean estimates for 6-minute walk distance and emPHasis-10 from PHANTOM (Pulmonary Hypertension and Anastrozole) (A and B) and Penn (Pennsylvania) Cohort (C and D) with activity levels based on regression models adjusted for age, sex, body mass index, World Health Organization functional class, and use of parenteral prostacyclin analog therapy. 6MWD = 6-minute walk distance; E-10 = emPHasis-10.
Validation of Activity Phenotypes
The Penn cohort included 60 women. The mean age was 50, idiopathic PAH was the most common etiology, and 35% were receiving parenteral prostacyclin analogs (Table 3). The patients were younger, all women, and more commonly in WHO functional class III. Patients wore the ActiGraph for an average of 15.8 hours each day, consistent with somewhat lower adherence considering this sample was instructed to wear the ActiGraph 24 h/d. Wear times varied greatly among patients. (Figure E1B). Four patients had days with fewer than 5 hours of wear time, three patients with 1 day each and one patient with 2 days, excluded from analyses. E-10 scores and 6-minute walk distances were similar in both cohorts. Accelerometry variables were systematically higher in the Penn cohort, as expected with the device worn on the wrist compared with the waist in PHANTOM (41, 42). Patients spent a majority of their time being sedentary with a small amount in light activity and no vigorous activity. Similar to PHANTOM results, there was significant variability in activity noted in this cohort depending on time of the day (Figure 1B).
Table 3.
Patient characteristics (Penn Cohort)
| n = 60 | |
|---|---|
| Age, yr | 50 ± 18 |
| Sex, female, % | 100 |
| Body mass index, kg/m2 | 28 ± 6.2 |
| Race, % | |
| White | 58 |
| African American | 32 |
| Other | 10 |
| PAH etiology, % | |
| Idiopathic/heritable | 50 |
| Connective tissue disease | 31 |
| Other | 19 |
| PAH medications, % | |
| Only oral | 56 |
| Inhaled ± oral | 9 |
| Parenteral prostacyclin analog ± oral | 35 |
| WHO functional class, % | |
| I | 9 |
| II | 46 |
| III | 45 |
| emPHasis-10 | 19 ± 12 |
| 6-minute walk distance, m | 403 ± 129 |
| TAPSE, cm | 2.0 (1.7–2.2) |
| Accelerometry variables (per day) | |
| Vector magnitude counts | 1,860,000 ± 613,800 |
| Step counts | 7,960 ± 2,710 |
| Sedentary, min | 333 (255–396) |
| Light activity, min | 236 (207–260) |
| Moderate activity, min | 16 (12–29) |
Definition of abbreviations: PAH = pulmonary arterial hypertension; Penn = Pennsylvania; TAPSE = tricuspid annular plane systolic excursion; WHO = World Health Organization.
Data are summarized as mean ± SD or median (interquartile range).
We used a K-nearest neighbors algorithm to project accelerometer features from the Penn cohort onto the PCs derived from PHANTOM. Three clusters with similar distribution of activity states were identified in this cohort. (Figure 5B and Table 2). There were no significant differences between the age or etiology of PAH among the clusters in the Penn cohort. Cluster assignment in this cohort was again independently associated with 6-minute walk distance, where patients who were more active had higher 6-minute walk distance (Figure 6C), but there were no differences in E-10 (Figure 6D). There was no significant association between TAPSE and cluster assignment.
Discussion
In a multicenter sample from a clinical trial of men and postmenopausal women with PAH, better RV function was associated with increased levels of accelerometer-measured daily physical activity. A 1-SD increase in TAPSE was associated with an increase in vector magnitude counts by 34,000 units or 0.23 SDs. Higher levels of physical activity were associated with a greater 6-minute walk distance and better PAH-specific HRQoL, as defined by the E-10 score. With the use of novel features derived from accelerometers, pattern-based activity phenotypes were identified and reproducible in a second cohort of PAH patients. In both of these distinct study samples, the more physically active group had greater 6-minute walk distance. Subjects in both cohorts were sedentary for a majority of their time (8–10 h/d), with only minimal time spent in activities of moderate intensity (1–20 min/d).
Patients with PAH are frequently limited in their physical activity owing to dyspnea, fatigue, and, in more severe cases, syncope. Impairment in RV contractility leading to an inability to adequately augment cardiac output is thought to be the culprit that precipitates these symptoms (43). In animal models of PAH, improved RV function improves exercise capacity (44). Clinically, the 6-minute walk distance is most often used as a measure of exercise capacity to assess disease severity and manage therapy (1). However, it does not capture the patterns of or variability in daily physical activity. A decline in the 6-minute walk distance may reflect worsening of disease, but the ability to identify worsening is dependent, by design, on the in-person completion of this test. Another major concern regarding the 6-minute walk test is the presence of a “ceiling effect” in those with milder disease (45, 46). Accelerometry has the advantage of measuring physical activity in the patients’ free-living environment on a much more frequent basis and can be assessed longitudinally. Our study showed a strong association between daily activity levels, activity phenotypes, and the 6-minute walk distance. The use of accelerometry offers the opportunity to objectively capture real-time changes in daily activity levels, a potentially meaningful tool that may detect early signs of disease worsening, prompting further clinical assessments.
Exercise capacity is an important prognostic indicator in PAH (1). The 6-minute walk distance correlates well with peak oxygen consumption (47) and is used widely as an endpoint in clinical trials of PAH therapies (48). Our study is the first to examine the relationship between RV systolic function and granular metrics of accelerometry to our knowledge that reflect patients’ daily activity in a multicenter cohort. We found that better RV systolic function measured by TAPSE was associated with higher daily physical activity. The associations of other measures of RV systolic function with physical activity were nonsignificant but were limited by missing data (Table E3).
Measurements of daily physical activity have been used to assess patients’ performance status in several other diseases, such as fibromyalgia, hypertension, chronic obstructive pulmonary disease, and lung cancer (49–52). In left-heart failure, reduced physical activity measured by accelerometry has been associated with worse functional status and lower HRQoL scores. However, investigations into the relationship of physical activity with outcome measures in PAH are limited to single-center cohorts with relatively small sample sizes and variability in data collection and processing. These studies in PAH have focused primarily on the association of step counts with clinical measures. Although step counts are an easily understood measure, they are not equitable across devices; step-counting algorithms are proprietary (and usually not publicly available) and use different thresholds to determine acceleration in different devices (53). Furthermore, step-counting accuracy is low in patients with chronic conditions and low walking speeds (54).
In our study, we utilized granular accelerometer output data measures in addition to step counts and showed a strong association between daily physical activity, activity phenotypes, and HRQoL scores in the multicenter PHANTOM cohort. The relationship between activity phenotypes and HRQoL was not seen in the single-center Penn Cohort. Although this cohort consisted of only women, it was thought to be representative of PAH epidemiology, with the disease being more prevalent in women. However, the somewhat younger all-female study population in this cohort could perhaps explain our findings. PHANTOM included an older population with PAH than other studies because of our exclusion of premenopausal females. These different age groups may have different mental or emotional factors that impact the E-10 in addition to cardiopulmonary disease. Difference in site of device wear may also have been a contributor. Alternatively, we speculate that a more likely explanation is the use of summary metrics for our analyses. In this study, patients wore ActiGraphs for 7 days. Total activity metrics were computed by summing daily levels and then using median values across the study visit duration for each participant. Although this use of median values helped identify overall patterns, we speculate that the granular data that captures between-day and within-day variability in physical activity may be pertinent.
Limitations
Our study has several limitations. Our cohorts included a relatively small number of patients in each cohort with differences in age and sex between the two cohorts. There was a high degree of missingness of RV systolic measures other than TAPSE. However, there appeared to be trends in the same direction. The two cohorts used different sites for device placement, one worn at the hip (PHANTOM) and the other the wrist (Penn). Prior studies have shown significant correlations between the measures obtained by both devices, although the wrist-based device tends to register higher overall counts attributable to frequent hand movements, as we also noted in our cohort (Figure 1) (41, 42). Despite these limitations, the identification of similar patterns of activity among the two PAH cohorts is informative.
Conclusions
Overall, our study suggests that patients with PAH segregate into distinct groups based on their physical activity levels, which link to clinical measures like the 6-minute walk distance and HRQoL. It is possible that future trials could use “clustering” to enrich enrollment in clinical trials. Accelerometry may be a promising tool to assess daily physical activity in patients with PAH.
Footnotes
Supported by National Institutes of Health grants R01-HL134905 and K24-HL103844 (S.M.K.), K23-NR014885 (L.A.M.), and T32-HL007891 (J.M.).
Author Contributions: J.M., S.H., S.M.K., and A.S. were involved in conceptualization of this project. J.M., H.S., S.H., N.A.-N., L.A.M., S.M.K., and A.S. were involved in data collection and analysis. All authors were involved in the generation of this manuscript and its approval. J.M., S.M.K., and A.S. are guarantors of the paper and take responsibility for the integrity of the work as a whole.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J . 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 2. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc . 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 3. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep . 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 4. Chia KSW, Brown K, Kotlyar E, Wong PKK, Faux SG, Shiner CT. ‘Tired, afraid, breathless….’ An international survey of the exercise experience for people living with pulmonary hypertension. Pulm Circ . 2020;10:2045894020968023. doi: 10.1177/2045894020968023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seefeldt V, Malina RM, Clark MA. Factors affecting levels of physical activity in adults. Sports Med . 2002;32:143–168. doi: 10.2165/00007256-200232030-00001. [DOI] [PubMed] [Google Scholar]
- 6. Mainguy V, Provencher S, Maltais F, Malenfant S, Saey D. Assessment of daily life physical activities in pulmonary arterial hypertension. PLoS One . 2011;6:e27993. doi: 10.1371/journal.pone.0027993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pugh MEB, Buchowski MS, Robbins IM, Newman JN, Hemnes AR. Physical activity limitation as measured by accelerometry in pulmonary arterial hypertension. Chest . 2012;142:1391–1398. doi: 10.1378/chest.12-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matura LA, Shou H, Fritz JS, Smith KA, Vaidya A, Pinder D, et al. Physical activity and symptoms in pulmonary arterial hypertension. Chest . 2016;150:46–56. doi: 10.1016/j.chest.2016.02.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halliday SJS, Shi H, Brittain EL, Hemnes AR. Reduced free-living activity levels in pulmonary arterial hypertension patients. Pulm Circ . 2018;9:2045894018814182. doi: 10.1177/2045894018814182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sehgal S, Chowdhury A, Rabih F, Gadre A, Park MM, Li M, et al. STep-count using an Accelerometer for Monitoring PAH—STAMP Study group Counting steps: a new way to monitor patients with pulmonary arterial hypertension. Lung . 2019;197:501–508. doi: 10.1007/s00408-019-00239-y. [DOI] [PubMed] [Google Scholar]
- 11. Fritz JS, Blair C, Oudiz RJ, Dufton C, Olschewski H, Despain D, et al. Baseline and follow-up 6-min walk distance and brain natriuretic peptide predict 2-year mortality in pulmonary arterial hypertension. Chest . 2013;143:315–323. doi: 10.1378/chest.12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sitbon O, Gomberg-Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J . 2019;53:1801908. doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J . 2005;26:858–863. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 14. Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med . 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 15. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16. Karas M, Bai J, Strączkiewicz M, Harezlak J, Glynn NW, Harris T, et al. Accelerometry data in health research: challenges and opportunities. Stat Biosci . 2018;11:210–237. doi: 10.1007/s12561-018-9227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathie MJ, Coster AC, Lovell NH, Celler BG. Accelerometry: providing an integrated, practical method for long-term, ambulatory monitoring of human movement. Physiol Meas . 2004;25:R1–R20. doi: 10.1088/0967-3334/25/2/r01. [DOI] [PubMed] [Google Scholar]
- 18. Schutz Y, Weinsier RL, Hunter GR. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obes Res . 2001;9:368–379. doi: 10.1038/oby.2001.48. [DOI] [PubMed] [Google Scholar]
- 19. Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med . 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 20. Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J . 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr . 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 22. Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, et al. American Thoracic Society Assembly on Pulmonary Circulation Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American Thoracic Society research statement. Am J Respir Crit Care Med . 2018;198:e15–e43. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ware JE., Jr SF-36 health survey update. Spine . 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 24. Lewis RA, Armstrong I, Bergbaum C, Brewis MJ, Cannon J, Charalampopoulos A, et al. EmPHasis-10 health-related quality of life score predicts outcomes in patients with idiopathic and connective tissue disease-associated pulmonary arterial hypertension: results from a UK multicentre study. Eur Respir J . 2020;57:2000124. doi: 10.1183/13993003.00124-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yorke J, Corris P, Gaine S, Gibbs JS, Kiely DG, Harries C, et al. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J . 2014;43:1106–1113. doi: 10.1183/09031936.00127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zlupko M, Harhay MO, Gallop R, Shin J, Archer-Chicko C, Patel R, et al. Evaluation of disease-specific health-related quality of life in patients with pulmonary arterial hypertension. Respir Med . 2008;102:1431–1438. doi: 10.1016/j.rmed.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 27. Chin KM, Gomberg-Maitland M, Channick RN, Cuttica MJ, Fischer A, Frantz RP, et al. Psychometric validation of the pulmonary arterial hypertension-symptoms and impact (PAH-SYMPACT) questionnaire: results of the SYMPHONY trial. Chest . 2018;154:848–861. doi: 10.1016/j.chest.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 28. Mehta S, Sastry BKS, Souza R, Torbicki A, Ghofrani H-A, Channick RN, et al. Macitentan improves health-related quality of life for patients with pulmonary arterial hypertension: results from the randomized controlled SERAPHIN trial. Chest . 2017;151:106–118. doi: 10.1016/j.chest.2016.08.1473. [DOI] [PubMed] [Google Scholar]
- 29. Chen H, Taichman DB, Doyle RL. Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proc Am Thorac Soc . 2008;5:623–630. doi: 10.1513/pats.200802-020SK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Domelen DR, Pittard WS, Harris TB.2014. https://github.com/vandomed/nhanesaccel
- 31. Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003-2006. Prev Chronic Dis . 2012;9:E113. doi: 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colley R, Connor Gorber S, Tremblay MS. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep . 2010;21:63–69. [PubMed] [Google Scholar]
- 33. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc . 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 34. Huang JZ. An introduction to statistical learning: with applications in R by Gareth James, Trevor Hastie, Robert Tibshirani, Daniela Witten. J Agric Biol Environ Stat . 2014;19:556–557. [Google Scholar]
- 35. Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Philos Trans- Royal Soc, Math Phys Eng Sci . 2016;374:20150202. doi: 10.1098/rsta.2015.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ringnér M. What is principal component analysis? Nat Biotechnol . 2008;26:303–304. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 37. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med . 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zaihra T, Walsh CJ, Ahmed S, Fugère C, Hamid QA, Olivenstein R, et al. Phenotyping of difficult asthma using longitudinal physiological and biomarker measurements reveals significant differences in stability between clusters. BMC Pulm Med . 2016;16:74. doi: 10.1186/s12890-016-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Filipow N, Davies G, Main E, Sebire NJ, Wallis C, Ratjen F, et al. Unsupervised phenotypic clustering for determining clinical status in children with cystic fibrosis. Eur Respir J . 2021;58:2002881. doi: 10.1183/13993003.02881-2020. [DOI] [PubMed] [Google Scholar]
- 40. Wang C, Long Y, Li W, Dai W, Xie S, Liu Y, et al. Exploratory study on classification of lung cancer subtypes through a combined K-nearest neighbor classifier in breathomics. Sci Rep . 2020;10:5880. doi: 10.1038/s41598-020-62803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clevenger KA, Pfeiffer KA, Montoye AHK. Cross-generational comparability of hip- and wrist-worn ActiGraph GT3X+, wGT3X-BT, and GT9X accelerometers during free-living in adults. J Sports Sci . 2020;38:2794–2802. doi: 10.1080/02640414.2020.1801320. [DOI] [PubMed] [Google Scholar]
- 42. Mandigout S, Lacroix J, Perrochon A, Svoboda Z, Aubourg T, Vuillerme N. Comparison of step count assessed using wrist- and hip-worn Actigraph GT3X in free-living conditions in young and older adults. Front Med (Lausanne) . 2019;6:252. doi: 10.3389/fmed.2019.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naeije R. Treatment of right heart failure on pulmonary arterial hypertension: is going left a step in the right direction? Eur Respir Rev . 2010;19:4–6. doi: 10.1183/09059180.00008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Handoko ML, Lamberts RR, Redout EM, de Man FS, Boer C, Simonides WS, et al. Right ventricular pacing improves right heart function in experimental pulmonary arterial hypertension: a study in the isolated heart. Am J Physiol Heart Circ Physiol . 2009;297:H1752–H1759. doi: 10.1152/ajpheart.00555.2009. [DOI] [PubMed] [Google Scholar]
- 45. Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, et al. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J . 2007;153:1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 46. Frost AE, Langleben D, Oudiz R, Hill N, Horn E, McLaughlin V, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol . 2005;43:36–39. doi: 10.1016/j.vph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 47. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J . 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 48. McLaughlin VV, Badesch DB, Delcroix M, Fleming TR, Gaine SP, Galiè N, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol . 2009;54:S97–S107. doi: 10.1016/j.jacc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 49. Landis CA, Frey CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JL. Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia. Nurs Res . 2003;52:140–147. doi: 10.1097/00006199-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 50. Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension . 1999;34:685–691. doi: 10.1161/01.hyp.34.4.685. [DOI] [PubMed] [Google Scholar]
- 51. Bauldoff GS, Ryan-Wenger NA, Diaz PT. Wrist actigraphy validation of exercise movement in COPD. West J Nurs Res . 2007;29:789–802. doi: 10.1177/0193945906297371. [DOI] [PubMed] [Google Scholar]
- 52. Fujisawa D, Temel JS, Greer JA, El-Jawahri A, Traeger L, Jacobs JM, et al. Actigraphy as an assessment of performance status in patients with advanced lung cancer. Palliat Support Care . 2019;17:574–578. doi: 10.1017/S1478951518001074. [DOI] [PubMed] [Google Scholar]
- 53. John D, Morton A, Arguello D, Lyden K, Bassett D. “What is a step?” Differences in how a step is detected among three popular activity monitors that have impacted physical activity research. Sensors (Basel) . 2018;18:1206. doi: 10.3390/s18041206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fokkema T, Kooiman TJ, Krijnen WP, VAN DER Schans CP, DE Groot M. Reliability and validity of ten consumer activity trackers depend on walking speed. Med Sci Sports Exerc . 2017;49:793–800. doi: 10.1249/MSS.0000000000001146. [DOI] [PubMed] [Google Scholar]