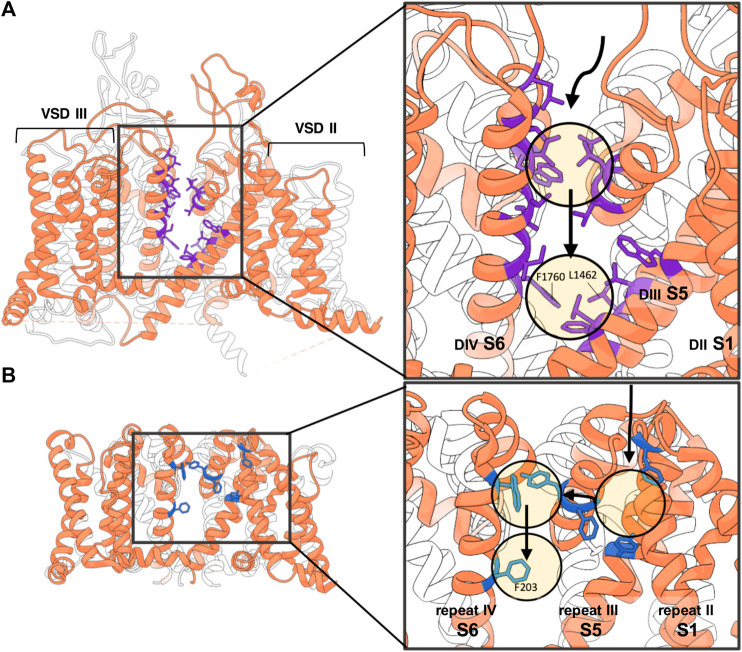
FIGURE 4.
Non-membranous lipophilic LA access via side fenestrations. (A) In the hydrophobic access pathway to the pore of the hNav1.5 homology model according to the simulations of (Nguyen et al., 2019) the LA molecule is stabilized along its trajectory by the following residues, colored in purple: L1338, L1342, and W1345 (in DIIIS5); L1410, L1413, and Q1414 (in P1 helix of DIII); L1462 and F1465 (in DIIIS6); W1713, L1717, and L1721 (in P2 helix of DIV); and I1749, T1753, I1756, and I1757 (in DIVS6); and at the entrance of the fenestration F1760 (in DIVS6) and L1462 (in DIIIS6) (Nguyen et al., 2019). Numberings are based on hNav1.5 sequence. (B) Lipohilic pathway of benzocaine accessing the pore domain of NavAb via the side fenestration. Along its trajectory it encounters low-energy hydrophobic pockets formed by the following residues colored in blue: F37, F142, F167, Y168, W195 and F203 (Boiteux et al., 2014). Numberings are based on NavAb sequence.