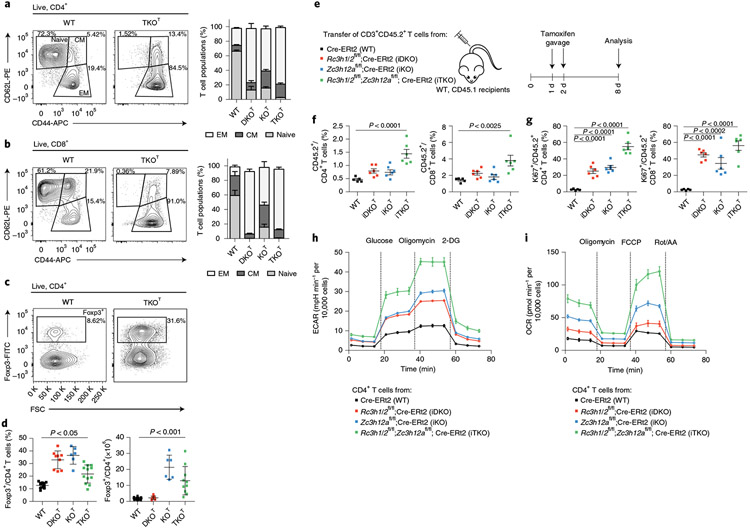
Fig. 1 ∣. Roquin-1/2 and Regnase-1 maintain quiescence of T cells.
a–d, Flow cytometry analysis of CD4+ (a), CD8+ (b) subpopulations (WT, n = 9; DKOT and KOT, n = 6; TKOT, n = 5 mice analyzed in at least three independent experiments) and Treg cells (c,d) (WT, n = 15; DKOT, n = 9; KOT, n = 6; TKOT, n = 10 mice analyzed in at least three independent experiments) from spleens of 6–8-week-old WT, DKOT, KOT and TKOT mice. CM, central memory; EM, effector memory; WT, wild type. e, CD45.2+CD3+ T cells from WT, iDKO, iKO and iTKO mice were adoptively transferred into WT CD45.1+ mice. Mice were treated with tamoxifen by oral gavage to induce deletion of floxed alleles. f,g, Frequency (f) and proliferation (g) of CD45.2+, CD4+ and CD45.2+, CD8+ T cells were analyzed by flow cytometry on day 8 after transfer (n = 6 biological replicates). h,i, CD4+ T cells from WT, iDKO, iKO and iTKO mice were treated with 4′-OH-tamoxifen in vitro to induce deletion of floxed alleles. T cells were kept under type 1 helper T cell conditions and expanded with IL-2-containing medium for 2 d. IL-2 was withdrawn overnight followed by restimulation with anti-CD3/28 before glycolytic (h) and mitochondrial stress testing (i) (n = 3 independent experiments). 2-DG, 2-deoxyglucose; Rot, rotenone; AA, antimycin. Data are presented as mean ± s.e.m., analyzed by one-way analysis of variance (ANOVA) with Bonferroni (d) or Dunnett’s (f,g) post hoc test.