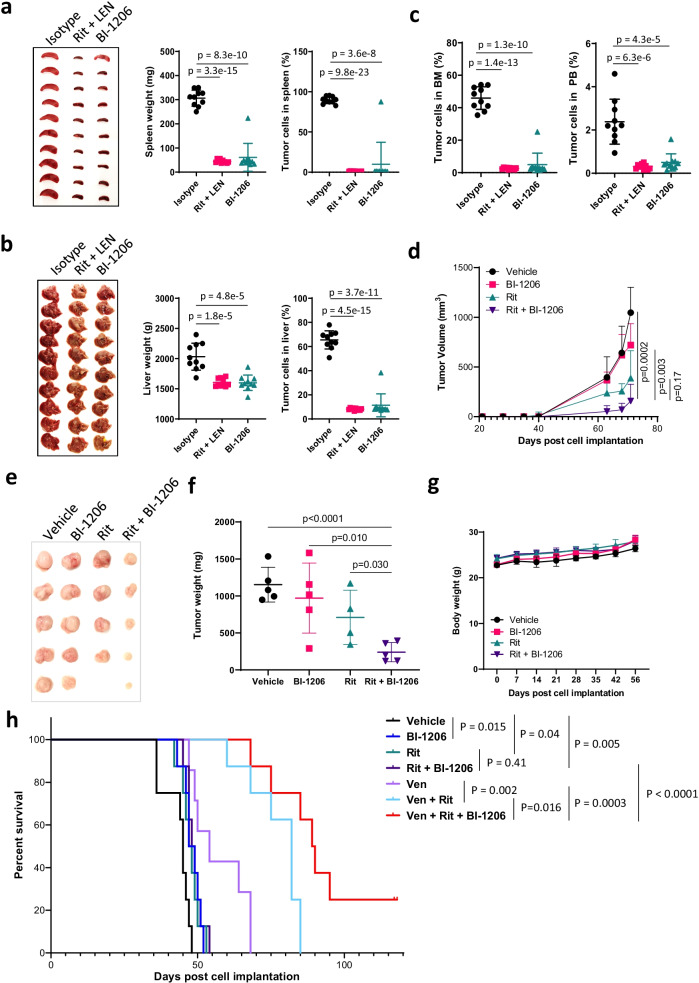
Fig. 2.
BI-1206 enhanced the in vivo efficacy of rituximab-based therapy in various MCL PDX models with multi-resistance in addition to intrinsic anti-MCL activity. a–c An ibrutinib-venetoclax dual resistant MCL PDX model (PDX-A) was derived from a patient with MCL with subsequent relapse from ibrutinib and venetoclax therapy. The PDX cells were inoculated intravenously into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. At 2 weeks post-cell inoculation, the mice (n = 10 per group) were randomly grouped and treated with isotype control (10 mg/kg, twice a week), BI-1206 (10 mg/kg, twice a week) or Rituximab (Rit, 10 mg/kg, twice a week) plus Lenalidomide (LEN, 2 mg/kg, daily). At the end of the experiment, the mice were euthanized, and spleen, liver, bone marrow and peripheral blood were collected. a, b The mouse spleen (a) and liver (b) were imaged (left panels) and weighed (middle panels). The cells in the spleen and liver were isolated and subject to flow analysis by dual staining with CD5 and CD20 antibodies. The percentage of CD5+CD20+ cells representing MCL tumor cells were plotted (right panels). c The cells in the bone marrow (left panel) and peripheral blood (right panel) were isolated and subject to flow analysis by dual staining with CD5 and CD20 antibodies. The percentage of CD5+CD20+ cells representing MCL tumor cells were plotted. d–g A rituximab-ibrutinib-CAR T-triple resistant MCL PDX model (PDX-B) was derived from a patient with MCL with subsequent relapse from rituximab, ibrutinib and CAR T-therapy. The PDX cells were inoculated subcutaneously into NSG mice. At 2 weeks post-cell inoculation, the mice (n = 5 per group) were randomly grouped and treated with isotype control (10 mg/kg, twice a week), BI-1206 (10 mg/kg, twice a week), Rituximab (Rit, 10 mg/kg, twice a week) or Rit + BI-1206 combo. One mouse in the rituximab-treated group died due to treatment-irrelevant technical event. Tumor size (d) and mouse body weight (g) were measured every week. At the end of the experiment, the mice were euthanized, and subcutaneous tumors were collected, imaged (e) and weighed (f). h An ibrutinib-rituximab-venetoclax triple-resistant MCL PDX (PDX-C) model was derived from a patient with MCL with relapse from ibrutinib plus rituximab therapy and venetoclax therapy. The PDX cells were inoculated subcutaneously into NSG mice. At 2 weeks post-cell inoculation, the mice (n = 8 per group) were randomly grouped and treated with vehicle control, BI-1206 (10 mg/kg, twice a week), Rituximab (Rit, 10 mg/kg, twice a week), Rituximab + BI-1206 (Rit + BI-1206), venetoclax (Ven, 50 mg/kg, daily), venetoclax + Rituximab (Ven + Rit) or venetoclax + Rituximab + BI-1206 (Ven + Rit + BI-1206). Mouse survival was monitored and plotted