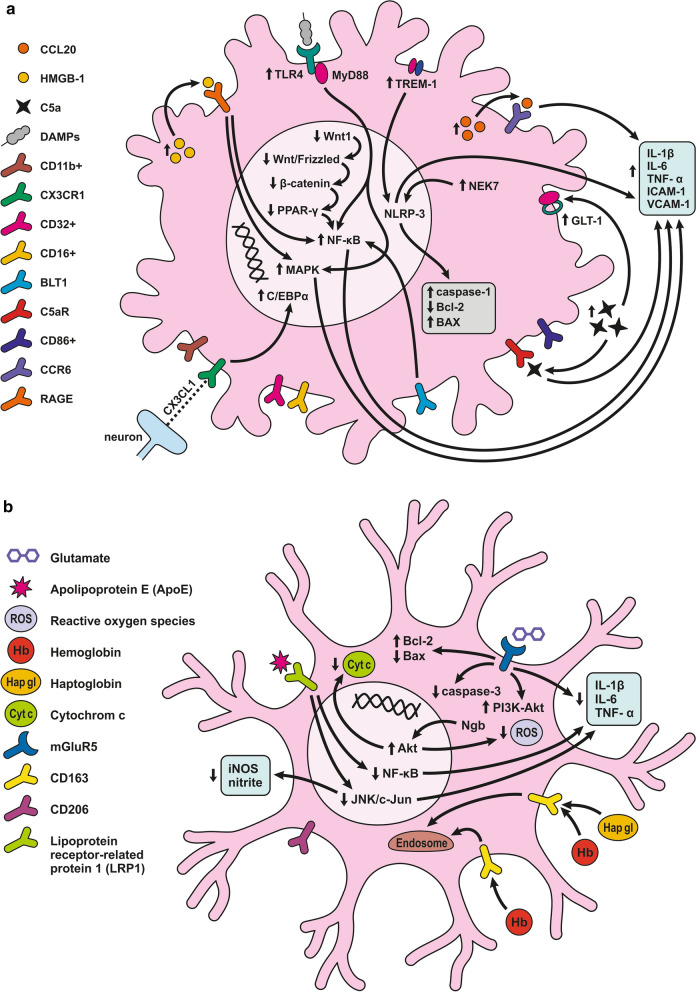
Fig. 6.
Reaction of microglia to SAH. a Activated microglia-induced inflammation. Presence of CD11b+/ CD16+/ CD32+/ CD86+ on microglia promotes inflammatory activation of M1 type. TLR4 and BLT1 activation upregulate NF-κB, initiating inflammatory cytokine production and resulting in EC and neuronal apoptosis. Upregulated NEK7 and TREM-1 activate NLRP3, promoting caspase-1 and IL-1β maturation, Bax upregulation, and Bcl-2 reduction. CCR6 also promotes inflammation after the increased release of CCL20. C5a receptor responds to released C5a and also contributes to the increased production of inflammatory cytokines. Downregulation of the CX3CL1/CX3CR1 axis causes an increase in C/EBPα, resulting in pro-inflammatory responses. RAGE is activated via HMGB1, causing MAPK upregulation, thus NF-κB activation, and brain inflammation. Wnt1 downregulation suppresses the Wnt/Frizzled signaling pathway, which leads to β-catenin reduction. Downregulated PPAR-γ then results in inflammatory responses by NF-κB activation. b Protective role of microglia after SAH. Interaction of CD163 with Hb results in Hb internalization to endosomes for degradation into heme, peptides, and amino acids. mGluR5 regulates glutamate detoxification and reduces pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Activation of mGluR5 also leads to Bcl-2 upregulation and the downregulation of Bax and active caspase-3. PI3K-Akt pathway activation and subsequent cell survival are regulated by mGluR5. The Akt signaling pathway is activated by neuroglobin functioning as a ROS scavenger and Cyt c release inhibitor. LRP1 activation by ApoE downregulates the NF-κB inflammatory cascade and inhibits the JNK/c-Jun pathway, suppresses microglial activation, and inhibits iNOS and nitrite accumulation