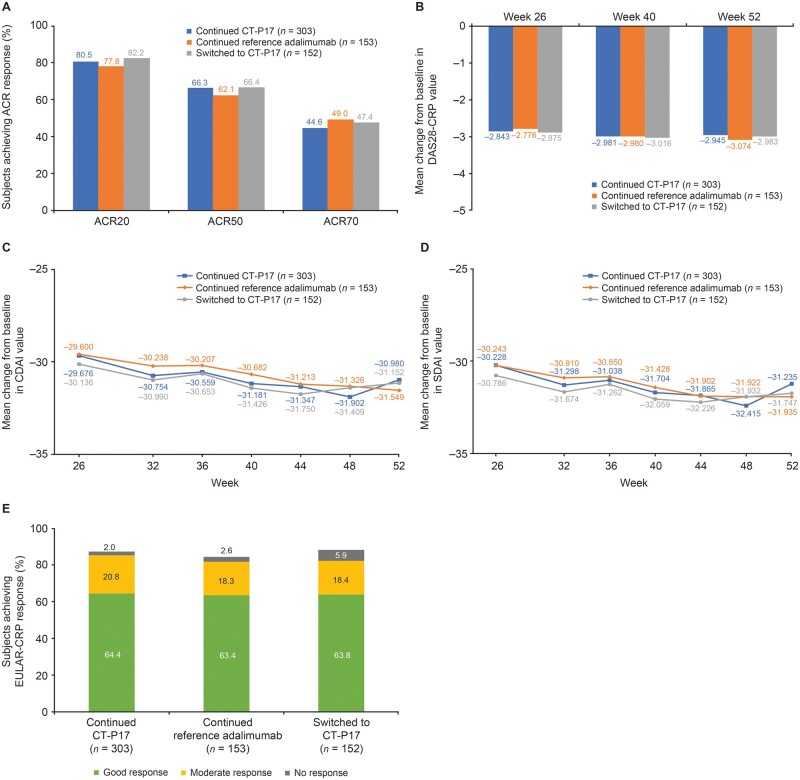
Fig. 3.
Clinical efficacy during treatment period 2 (intention-to-treat population—treatment period 2 subset)
(A) ACR response rates at week 52; (B) Mean change from baseline in DAS28-CRP value during treatment period 2; (C) Mean change from baseline in CDAI value during treatment period 2; (D) Mean change from baseline in SDAI value during treatment period 2; (E) EULAR-CRP response rates at week 52. ACR20/50/70: 20%/50%/70% improvement according to ACR criteria; CDAI: Clinical Disease Activity Index; DAS28-CRP: Disease Activity Score in 28 joints–CRP; SDAI: Simplified Disease Activity Index.