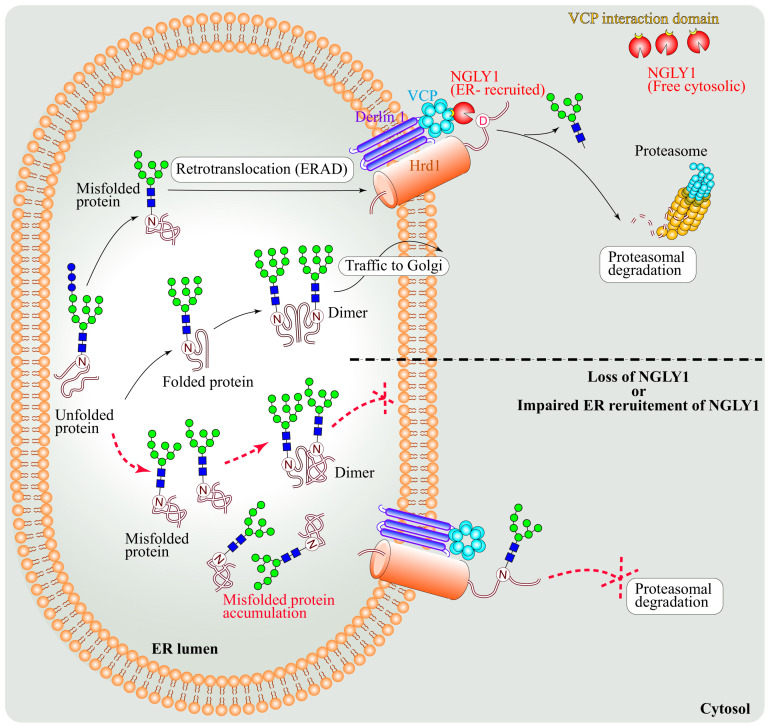
Figure 6.
Schematic representation showing that de-N-glycosylation of misfolded bone morphogenetic protein 4 (BMP4) by endoplasmic reticulum (ER) membrane-recruited NGLY1 promotes its retrotranslocation from ER to cytosol, where it undergoes proteasomal degradation. In the absence of NGLY1 or its impaired ER recruitment, misfolded BMP4 molecules cannot be retrotranslocated to the cytosol and are accumulated in the ER lumen, where they can potentially dimerize with properly folded BMP4 molecules and prevent the trafficking of BMP4 dimers from ER to the Golgi. N, asparagine; D, aspartic acid; VCP, Valosin-Containing protein; ERAD, Endoplasmic reticulum associated degradation.