Figure 4.
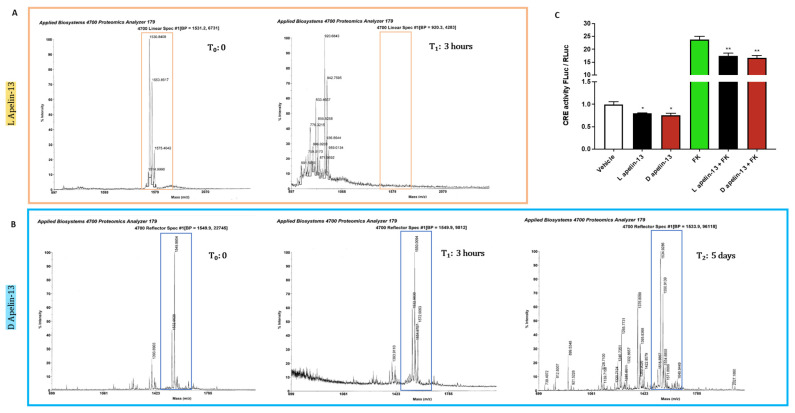
While L-apelin 13 is degraded in mouse serum in three hours, D-apelin 13 is stable for days. The peptide [Pyr1]-apelin 13 (MW 1533.81 g/mol) in L version (A) or D version of apelin 13 (B) were incubated at the same concentration in mouse serum at 37 °C. After different times from three hours to five days, a sample was analyzed using a MALDI-TOF mass spectrometer in linear mode. The molecular weights were compared with the original peptides at time 0. The highlighted peak that detects L-apelin 13 disappeared after 3 h (A), when additional peaks corresponding to lower molecular weights appear, indicating its fragmentation. Instead, D-apelin 13 was more stable in the mouse serum, because its peak (MW 1550 g/mol) persisted for five days (B). Myoblasts were transfected with a plasmid in which Firefly luciferase (FLuc) is under the control of cAMP responsive elements (CRE) and another for Renilla luciferase (RLuc) under the control of thymidine kinase promoter, to normalize the data. After 24 h, myoblasts were treated for 5 h with vehicle or 1 μM L-apelin 13 or D-apelin 13, in combination with vehicle or 50 μM forskolin and the ratio of FLuc/RLuc is plotted. L-apelin 13 and D-apelin 13 were equally able to reduce cAMP and forskolin-induced cAMP by about 25% in luciferase assays (C). Ordinary one-way ANOVA, * p ≤ 0.05, ** p ≤ 0.01, n = 6.