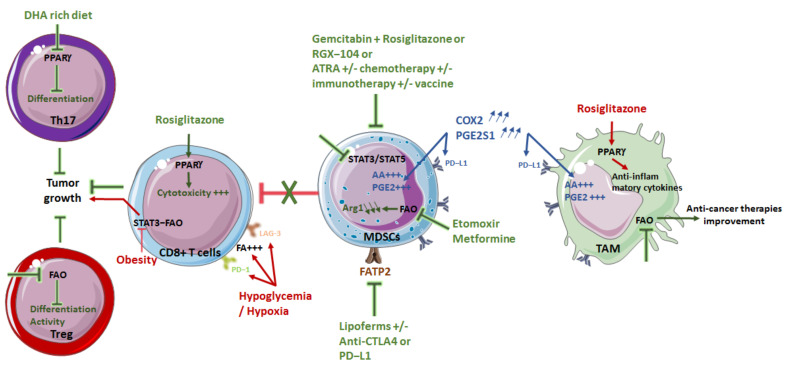
Figure 4.
Targeting immune cell lipid metabolism as a therapeutic strategy. PPAR agonist (rosiglitazone) with or without chemotherapy induces an increase of anti-inflammatory cytokines in M2 macrophages, an increase in CD8+ T-cell cytotoxicity as well as inhibition of MDSC immunosuppressive function. In contrast, inhibition of PPAR, following a DHA-rich diet, inhibits Th17 differentiation. All of these changes lead to a decrease of tumor progression. Overexpression of COX2 and PGE2S1 are also capable of targeting tumor growth by increasing arachidonic acid (AA), PGE2 production and PD-L1 expression both in MDSCs and in TAMS. FAO inhibitors also induce an alteration of MDSCs, TAM and Treg differentiation and function, leading to improvement of anti-cancer therapies. Lipoferms, an inhibitor of FATP2, with or without immunotherapies targeting CTLA4 or PD-L1, as well as inhibitors of STAT3/STAT5 induce a strong inhibition of MDSC function and decreased tumor growth. Conversely, inhibition of the leptin–STAT3–FAO pathway on CD8+ T cells, which occurrs in obese individuals, promotes tumor growth. Similarly, hypoglycemia and hypoxia lead to CD8+ T-cell inhibition through overexpression of PD-1 and LAG-3, which increases free FA around them.