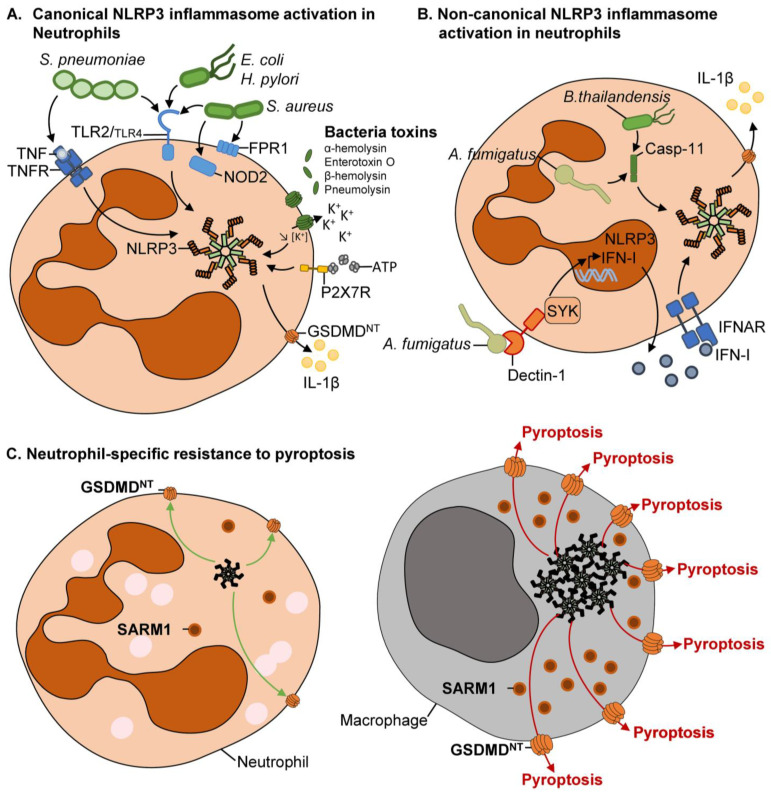
Figure 2.
Mechanism NLRP3 inflammasome activation in neutrophils. (A). Canonical NLRP3 inflammasome activation in neutrophils. Extracellular bacteria are mainly sensed by Toll-like receptor 2 (TLR2) and, in some cases, TLR4, Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) or formyl peptide receptor 1 (FPR1), leading to efficient priming. This first step can also be mediated indirectly by host factors such as TNF and TNFR engagement. The main NLRP3 inflammasome activation signals are dependent of bacterial toxins from Escherichia coli (e.g., α-hemolysin), Staphylococcus aureus (e.g., enterotoxin O), Streptococcus agalactiae (e.g., β-hemolysin), and Streptococcus aureus (e.g., pneumolysin). The damages they cause to the neutrophil lead to increased potassium (K+) efflux, extracellular ATP, and subsequent NLRP3 inflammasome assembly via P2X receptor 7 (P2X7R). (B). Non-canonical NLRP3 inflammasome activation in neutrophils. The non-canonical NLRP3 inflammasome is activated in response to Gram-negative bacteria (Burkholderia thailandensis) or filamentous fungi Aspergillus fumigatus. This triggers caspase-11 activation and then NLRP3 inflammasome activation. In response to A. fumigatus this pathway depends on Dectin-1, spleen tyrosine kinase (SYK), and type I interferon (IFN-I) signaling. (C). Neutrophil-specific resistance to pyroptosis. Neutrophils are resistant to pyroptosis likely because they produce low ASC, caspase-1, and sterile alpha and TIR motif-containing 1 (SARM1) proteins as compared with macrophages. Indeed, SARM1 seems to be a key regulator of the rate of Gasdermin D (GSDMD) pore formation at the plasma membrane to mediate pyroptosis. Thus, low SARM1 production by neutrophils may lead to decreased cell lysis.