Figure 4.
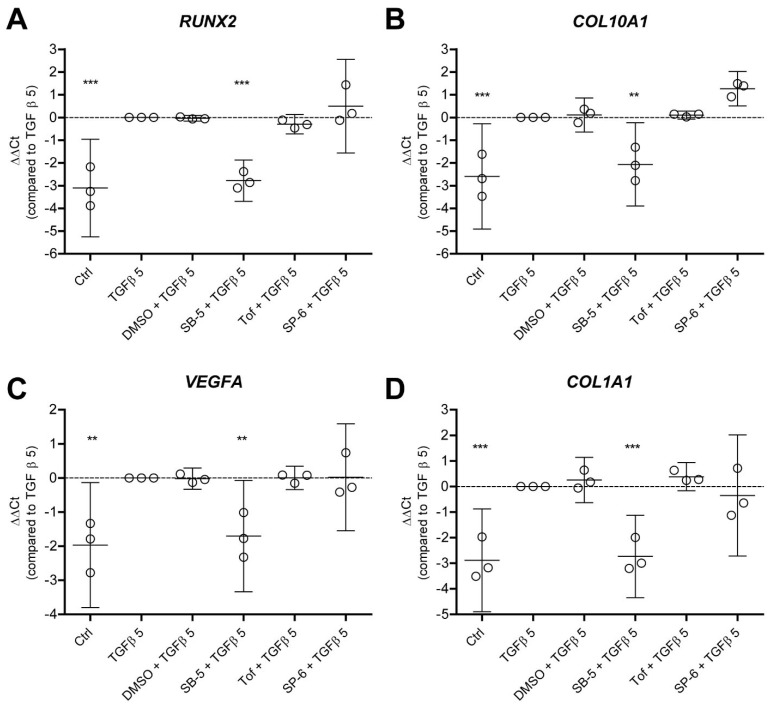
A hypertrophy-like phenotype in OA chondrocytes, induced by TGF-β, is dependent on ALK5 and is not reliant on JAK and JNK. Elevated levels of TGF-β that are comparable to those in synovial fluid of OA patients induce expression of hypertrophic factors in human OA chondrocytes of three different donors. This is defined by increased mRNA expression of (A) runt-related transcription factor 2 (RUNX2), (B) relative collagen type 10 (COL10A1), (C) vascular endothelial growth factor A (VEGFA) and (D) alpha-1 collagen type 1 (COL1A1) after stimulation with 5 ng/mL TGF-β1 for 48 h. Specific pathway inhibitors were used to target the SBE, SIE and AP1 pathways, which were also activated by these levels of TGF-β. Cells were pre-incubated with 5 µM SB-505124 (SB-5: ALK5 kinase activity inhibitor), 1 µM Tofacitinib (Tof: JAK inhibitor) or 10 µM SP-600125 (SP-6: JNK inhibitor) before stimulation with 5 ng/mL TGF-β1. Only blocking ALK5 kinase activity with SB-5 prevented the effect of TGF-β on hypertrophy genes, whereas inhibition of JAK and JNK did not. Data are plotted as mean ± 95% CI with each dot representing the average of three technical replicates in one chondrocyte donor (total of 3 different chondrocyte donors were used). Statistical analysis was performed using repeated measures one-way ANOVA with Bonferroni’s post hoc test: ** p ≤ 0.01; *** p < 0.001.