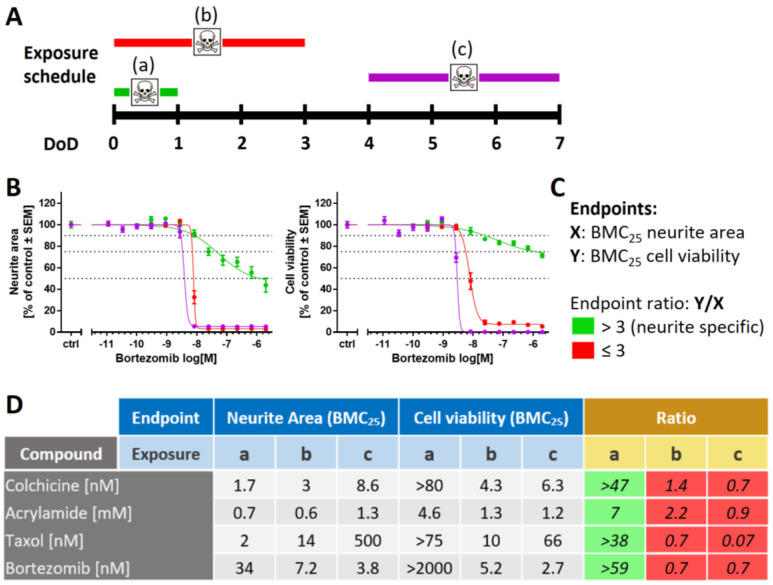
Figure 2.
Variation of the exposure schedule to assess compound toxicity. (A) Schematic representation of the applied exposure schedules with a 24 h treatment starting on DoD0 (a, standard PeriTox test, green), immediate 72 h treatment (b, DoD0-3, red), and delayed 72 h treatment (c, DoD4-7, purple). DoDx—day of differentiation, counting from thawing of frozen neural precursors on DoD0. (B) SBAD2-derived peripheral neurons were exposed to bortezomib according to the three exposure schedules. Effects on the neurite area and the cell viability were assessed. Data are expressed as the mean ± SEM of 3 independent experiments. (C) Prediction model for the classification of compound-induced effects. The concentrations relating to the benchmark response level of a 25% decrease of a test endpoint (BMC25) were calculated for both endpoints: neurite area (X) and cell viability (Y). A ratio of Y/X > 3 is classified as a “neurite-specific” compound effect (green). Y/X ≤ 3 marks effects that are “not neurite-specific”, and such effects were classified as “cytotoxic” (red). (D) BMC25 values were calculated for both test endpoints in all three exposure scenarios. Effects induced by colchicine, acrylamide, taxol, and bortezomib were classified according to the prediction model. Respective concentration–response curves are given in Figure S4.