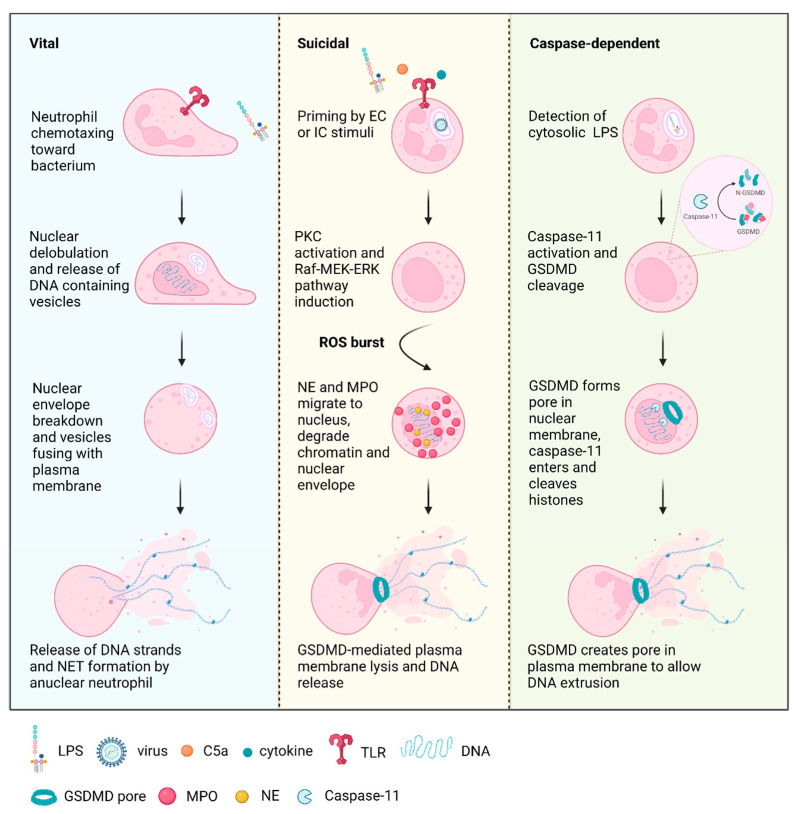
Figure 1.
Types of neutrophil extracellular trap (NET) formation. Vital NETosis is initiated by the detection of Gram-positive or Gram-negative bacteria via Toll-like receptor 2 (TLR2) [65,66], with the neutrophil continuing to migrate toward the bacterium. This is followed by nuclear condensation and release of DNA containing vesicles, which fuse with the outer membrane and release their contents to the extracellular space to form NETs. Nuclear envelope breakdown occurs to form anuclear neutrophils. Priming of suicidal NETosis occurs with the detection of cytokines such as tumor necrosis factor α (TNFα) and interleukin 8 (IL8) [5,67]; the complement component, C5a [68]; lipopolysaccharide (LPS) [5]; and viral glycoproteins [69,70,71], among other stimulants. This activates protein kinase C (PKC) [72] and the Raf-MEK-ERK pathway [73], while the nucleus begins to lose lobules [56]. These series of events are followed by an NADPH-mediated oxidative burst [56,57], which release contents from the granules such as neutrophil elastase (NE) and myeloperoxidase (MPO) into the cytoplasm [3,74]. NE and MPO migrate to the nucleus to act synergistically for the cleavage of histones and disintegration of the nuclear membrane [74]. NE also cleaves gasdermin D (GSDMD), enabling pore formation in the plasma membrane and NET release [59]. Similar to suicidal NETosis, caspase-dependent NETosis is also dependent on GSDMD for plasma membrane pore formation. It has been found to be triggered by cytosolic LPS [75], activating caspase-11, which cleaves GSDMD, leading to pore formation in the nuclear membrane, enabling the translocation of caspase-11 into the nucleus for histone cleavage [75]. GSDMD subsequently forms pores in the plasma membrane for NET extrusion [75]. Figure created using Biorender.