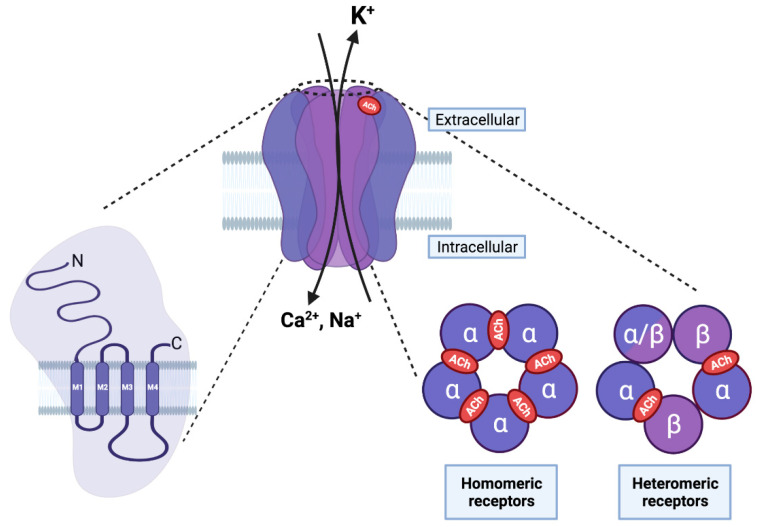
Figure 2.
Structure of nAChRs. On the left, each nAChR subunit is composed of an extracellular amino terminal portion, followed by three hydrophobic transmembrane domains (M1–M3), a large intracellular loop, a fourth transmembrane domain (M4), and an extracellular carboxy–terminus. In the middle, the pentameric arrangement of nAChR subunits is shown in an assembled receptor. The M2 transmembrane domain of the five subunits forms the central pore and possesses amino acids that are important for ion selectivity, permeability, and channel gating. On the right, five subunits can assemble to form homo- (five α subunits) or hetero-pentameric (α and β subunits) receptors. The orthosteric ligand binding site is formed between two α subunits (in red) in homomeric receptors, and between the α and β subunits in an heteromeric receptor. (Created with BioRender.com (accessed on 23 January 2022)).