Abstract
Study Design
Retrospective cohort study.
Objective
To determine safety and short-term outcomes of single-position lateral lumbar interbody fusion (LLIF) with bilateral posterior instrumentation and robotic assistance. The article also describes surgical technique considerations for the procedure.
Methods
20 patients underwent single-position LLIF with posterior instrumentation and robotic assistance. The patients were followed for a minimum of 3 months post-operatively.
Results
Average operative time was 211 ± 34 minutes, average blood loss was 51.25 ± 17 cc’s, and average length of stay was 1.4 ± .75 days. There were no intraoperative complications, readmissions, revision surgeries, and no incidence of hardware malposition. Significant improvement in pain and ODI scores was noted at 3 month follow up.
Conclusions
The study demonstrated safety and short-term clinical efficacy of minimally invasive single-position lateral lumbar interbody fusion with bilateral posterior instrumentation utilizing robotic assistance and navigation. There are certain surgical technique considerations that must be followed to ensure optimal surgical workflow and predictable outcomes.
Keywords: lumbar, degenerative disc disease, fusion, lumbar interbody fusion, pedicle screw, fixation, computer assisted navigation, low back pain, spinal navigation, XLIF, LLIF, single position, lateral fusion, robotic assisted surgery
Introduction
Minimally invasive lumbar fusion surgery (MIS) has been shown to be very beneficial for patients when compared to traditional open techniques. There are several MIS tissue-sparing approaches that have been shown to lead to lower blood loss, lower transfusion rates, shorter hospital stay, less pain, lower opioid intake, faster recovery, and lower cost of care.1-12
Open posterior lumbar fusion allows for direct visualization of anatomic landmarks for accurate decompression and hardware placement while minimizing the risk of nerve or blood vessel damage. Such direct visualization requires extensive dissection of muscles off the posterior spinal elements all the way out to the tips of the transverse processes. 13 This approach leads to significant blood loss, direct trauma to paraspinal musculature, interruption of blood and nerve supply to the musculature, creation of a large cavity just above the dura that may lead to seroma or hematoma formation, significant post-operative pain, and significant post-operative muscle stiffness due to denervation and scar tissue formation. The soft tissue disruption becomes even greater in multi-level procedures or in muscular or obese patients. 13
The intent of minimally invasive surgical techniques in spine is to achieve the same goals of nerve decompression, successful fusion, and deformity or instability correction while minimizing the collateral damage to the soft tissues. Common MIS approaches, however, severely limit or entirely eliminate surgeon’s ability to directly visualize important anatomic landmarks to ensure accurate hardware placement. Misplaced pedicle screws can lead to significant complications including nerve irritation or damage, blood vessel damage, facet joint violation, and inadequate fixation. This necessitates utilization of other methods to accurately assess the location of important structures and to ensure the correct position of instruments and implants during minimally invasive spine fusions. As the MIS techniques were being developed, surgeons mostly utilized biplanar intraoperative fluoroscopy for real-time verification of instrument and implant position. This led to significant increase in radiation exposure to the surgeon, OR staff, and the patient. The advent of three-dimensional image-guided navigation technology allowed for real-time verification of instrument position without the need for constant biplanar fluoroscopy. The latest step in the evolution of MIS spine surgery is utilization of rigid robotic arm and image-guided navigation to maintain proper trajectory for instrument and implant placement while minimizing soft tissue disruption. 15
Lateral lumbar interbody fusion (LLIF) is a minimally invasive lumbar fusion technique that utilizes lateral retroperitoneal transpsoas approach for discectomy and interbody spacer placement to achieve interbody fusion, restoration of disc height, deformity correction, and indirect decompression of spinal canal and neural foramina. 16 This technique is typically done with the patient in a lateral decubitus position. To achieve greater stability and deformity correction, posterior pedicle screws and rods are placed after the LLIF portion is done. Pedicle screw trajectory in lumbar spine is lateral to medial. This means that when the patient is in a lateral decubitus position, the contralateral screws are placed in an oblique and upward trajectory, which is very awkward and unfamiliar position for most surgeons. For this reason, the original LLIF technique was to place the interbody spacer with the patient in lateral decubitus position, close the flank incision, and then turn the patient prone and place the percutaneous pedicle screws. Repositioning the patient for the posterior instrumentation is time consuming, while keeping the patient under general anesthesia. It may also compromise instrument and implant sterility while all the surgical drapes must be taken off, new surgical table needs to be brought in, and the patient needs to be repositioned and re-draped. 14
Rigid robotic arm can increase the efficiency of LLIF procedure by making it easier for the surgeon to place posterior percutaneous pedicle screw instrumentation while keeping the patient in lateral decubitus position. In this small retrospective cohort study, we evaluated the feasibility of “single position lateral” technique utilizing computer navigation and robotic assistance for posterior instrumentation placement.
Methods
Between July 2020 and June 2021, 20 patients underwent single-position LLIF procedures at 1 or 2 levels at a single institution. 11 were female and 9 were male. Average age was 68.9 years. 13 patients had 1-level surgery and 7 patients had 2-level surgery. The data was analyzed retrospectively. Informed consent for data collection was obtained from all patients during pre-operative counseling. No institutional review board approval was needed for this retrospective review.
Excelsius GPS robotic and navigation system (Globus Medical, Audibon, Pennsylvania, USA) was utilized for posterior pedicle screw instrumentation in all cases. The system consists of a free-standing robotic arm and touchscreen display that is covered in translucent sterile plastic drape during the surgery. Intraoperative 3-D navigation is achieved using 2-detector infrared camera and infrared-reflective trackers attached to a stable skeletal structure in the patient, to every navigated instrument, and to the robotic arm. Desired screw trajectories are pre-planned on CT images of the spine that are obtained either pre-operatively or intraoperatively. During the surgery, the rigid robotic arm positions itself along the pre-planned pedicle screw trajectory just above the skin. Navigated instruments and hardware are then passed through the arm and into the pedicle. The robotic arm maintains rigid trajectory throughout the procedure preventing the instruments from deviating from the desired path [Figure 3].
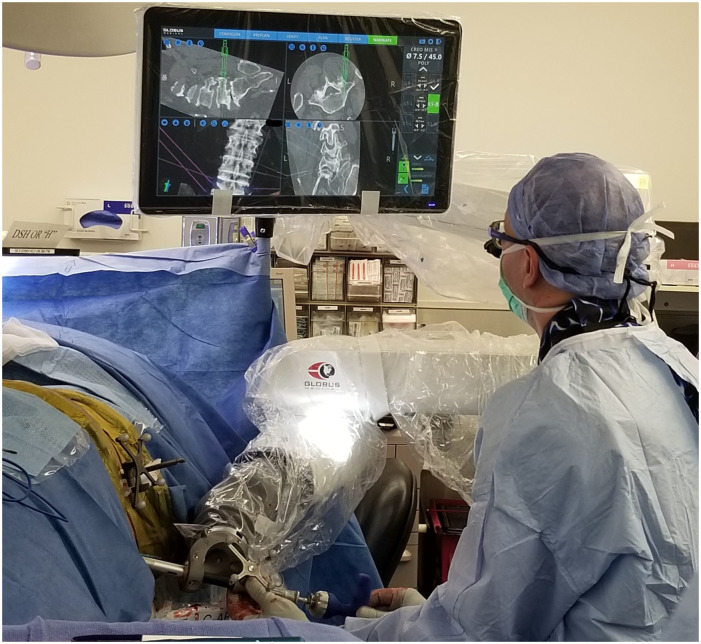
Figure 3.
Placement of the contralateral screw using a navigated screwdriver. Rigid robotic arm makes it easier to control proper trajectory for hardware placement while the monitor shows the position of the hardware relative to the spine and the planned trajectory in real time without the need to use fluoroscopy.
CT scan of the operative portion of the lumbar spine was obtained on all patients 1-3 weeks prior to surgery. The images were loaded into the Excelsius GPS system. Screw trajectories were planned pre-operatively using the navigation system software. At the beginning of the operation the intraoperative AP and lateral fluoroscopic images of each separate vertebra were obtained and merged with the pre-operative CT scan for segmental navigation to allow for accurate intraoperative guidance. Surveillance marker was used in all cases to ensure navigation accuracy throughout the case. Posterior pedicle screws were placed in percutaneous fashion using computer navigation through the rigid robotic arm that maintained trajectory along the pre-planned path. This was done before the anatomic relationship between the vertebrae was altered by placement of the interbody spacer(s). Proper screw placement was verified using intraoperative fluoroscopy in AP and lateral planes and by stimulating the screws with EMG neuromonitoring. Lateral lumbar interbody fusion was then performed utilizing the “direct look” technique using 22-mm wide interbody spacers at 1 or 2 levels. 17 The posterior rods were placed and locked into the pedicle screws as the last step, while maintaining the patient in the same lateral decubitus position. Proper hardware placement and bony alignment was confirmed using intraoperative fluoroscopy immediately before closing and using standing radiographs on post-operative day 1.
Operative time, intraoperative blood loss, length of stay, pain level on the 10-point visual analog scale, and ODI scores at 3 month follow up were recorded. The patients were followed for a minimum of 3 months post-operatively.
Results
Average operative time was 211 ± 34 minutes. Average blood loss was 51.25 ± 17 cc. Average length of stay was 1.4 ± .75 days. Most of the patients requiring 2-day stay had 2-level fusion. Only one patient stayed in the hospital longer than 2 days (4 days) due to post-operative left thigh pain related to the LLIF portion of the procedure. There were no intraoperative complications. There was no incidence of screw malposition requiring revision surgery. There were no readmissions or returns to the operating room for any reason. Average improvement in VAS at 3 months after surgery was 5.6 ± 2.6 points on the 1-10 scale. Average improvement in ODI scores at 3 months after surgery was 24.3 ± 18 points.
In 2 cases, the contralateral pedicle screw insertion was aborted due inability to achieve proper trajectory within the sterile field. In one case, unilateral instrumentation was utilized because the pedicles on the other side were too narrow to instrument. In all those cases expandable lateral interbody cages were used. When combined with unilateral instrumentation, these constructs were deemed to provide sufficient stability and repositioning and re-draping the patients to place the contralateral instrumentation or fixation was not deemed clinically necessary.
Discussion
The evolving capacity of minimally invasive surgical techniques to successfully achieve decompression and stabilization, as needed, aims to revolutionize the field of spinal surgery. The growing pressures from healthcare economics to provide value by minimizing operative time, length of stay, and post-operative complications are destined to continue. The value generated by MIS techniques is only maintained with the safe and effective placement of spinal instrumentation. This investigation demonstrated that robotic-assisted pedicle screw instrumentation is a safe and reliable technique to provide single-position (SP) posterior stabilization following LLIF in the lateral position.
There are several advantages to SP compared to dual-position (DP) circumferential fusion techniques reported in the literature. 18 The average operative time in our study of 205 min was substantially higher than other reports that utilized SP circumferential techniques. A retrospective study by Buckland et al 2 investigated outcomes following circumferential fusion by LLIF or Anterior Lumbar Interbody Fusion (ALIF) with posterior instrumentation reported an average operative time of 103.1 min in their SP group. 14 This finding is consistent with other investigations which reported even shorter average operative times, ranging from 93.3 to 98.4 min during single-position surgeries.19,20 The operative times observed in the SP group of the current investigation was longer and likely due to the fact the reported studies did not use robotic assistance for the posterior instrumentation portion of the procedure. Setting up the robotic arm requires additional workflow steps, and there is undoubtedly a learning curve for each level of the operative staff in using the technology. It is conceivable that with consistent usage, the workflow for robotic arm assistance will become more efficient.
In terms of blood loss, the current study results were comparable to reports in the literature following SP circumferential fusion which did not use a robotic arm for posterior pedicle screw instrumentation.14,19-21 Prone positioning may increase intra-abdominal pressure which can contribute to higher blood loss when using DP techniques. Multiple studies have reported blood loss to be a risk factor for increased length of stay.22,23 Several investigations have demonstrated that patients who undergo SP LLIF have lower average lengths of stay when compared to DP groups.14,20,21 In addition to lower estimated blood loss, the efficiency for SP LLIF decreases the time under general anesthesia. Time under general anesthesia may contribute to increased rates of post-operative ileus and prolonged hospitalization in patients who undergo DP LLIF. 14
In the current study, there were no mal-positioned screws following posterior instrumentation with the patient placed in the lateral decubitus position. While SP LLIF using traditional techniques is considered safe, the rates of screw malposition and pedicle breach has been reported to be as high as 5%. 24 To our knowledge, there is only one other report in the literature which investigated the reliability of robotic-assisted posterior instrumentation in SP LLIF. 25 As reported by Huntsman et al, 7 of 328 (2%) screws required repositioning, thereby supporting the high degree of precision observed in the current clinical investigation. The authors reported a greater mean estimated blood loss (117.4 mL) and length of stay (2.9 days) when compared to the current study results. This difference may be due to the fact that 35% of cases in the Huntsman et al. 25 study involved two-level posterior instrumentation. Moreover, the authors did not report post-operative follow up—highlighting the current report as the first to include short-term patient reported outcomes following SP lateral LLIF and posterior instrumentation.
Robotic-assisted technological platforms with integrated navigation have the potential to reduce human error secondary to prolonged cases and the technical/cognitive demands of complex spinal surgery.26,27 The Excelsius GPS is a relatively new robotic system (launched in 2017) and has demonstrated significantly higher levels of pedicle screw accuracy when compared to traditional techniques in cadaveric models.26,28 To our knowledge, this is the first clinical study which validates the accuracy of the Excelsius GPS system in vivo. Future studies with larger sample sizes of patients and long-term follow up are needed for improved comparisons of traditional MIS SP techniques and other robotic platforms.
Author’s Preferred Technique
There are several intraoperative technical considerations to optimize workflow when performing SP LLIF with posterior instrumentation.
1. The surgeon should confirm the pre-operative CT scan includes at least one level superior and inferior to the operative levels. The accuracy and reliability of the system may improve when more levels adjacent to the field of interest are registered in the software.
2. Pre-operative planning of screw trajectories with minimal obliquity is of paramount importance, particularly on the contralateral side to that of cage insertion. Failure to do so may result in screw starting points that are outside the prepped surgical field, particularly in patients with greater distance from skin to osseous structures [Figure 1].
3. In order to achieve a clear path for implantation of the contralateral instrumentation, the patient’s back should be positioned close to the edge of the operating table. Upper back and buttocks are typically aligned with the table edge, while maintaining the concavity of lumbar lordosis towards the table center. This positioning technique helps with proper spinal alignment and subsequent placement of contralateral screws, without interfering with intraoperative imaging.
4. One incision for all screws is recommended vs several small incisions. A single incision technique allows for single plane blunt dissection of soft tissues to the starting points and creates space for rod placement. This step prevents the rod from binding muscle fibers during insertion, allowing for greater operative efficiency.
5. Following positioning of the robotic arm to the desired trajectory, a side-cutting high-speed drill is utilized to create the initial opening in the cortex and tap the screw path (typically by under-tapping 1 mm) followed by screw insertion. [Figure 2, Figure 3] Force transducers localized on the robotic arm issue a warning if there is significant skiving from the planned trajectory. This feature is essential for correct and efficient contralateral screw placement.
6. Use of the rigid robotic arm throughout the implantation process is essential to maintain screw trajectory and accuracy, while limiting radiation exposure to the patient and surgeon [Figure 4].
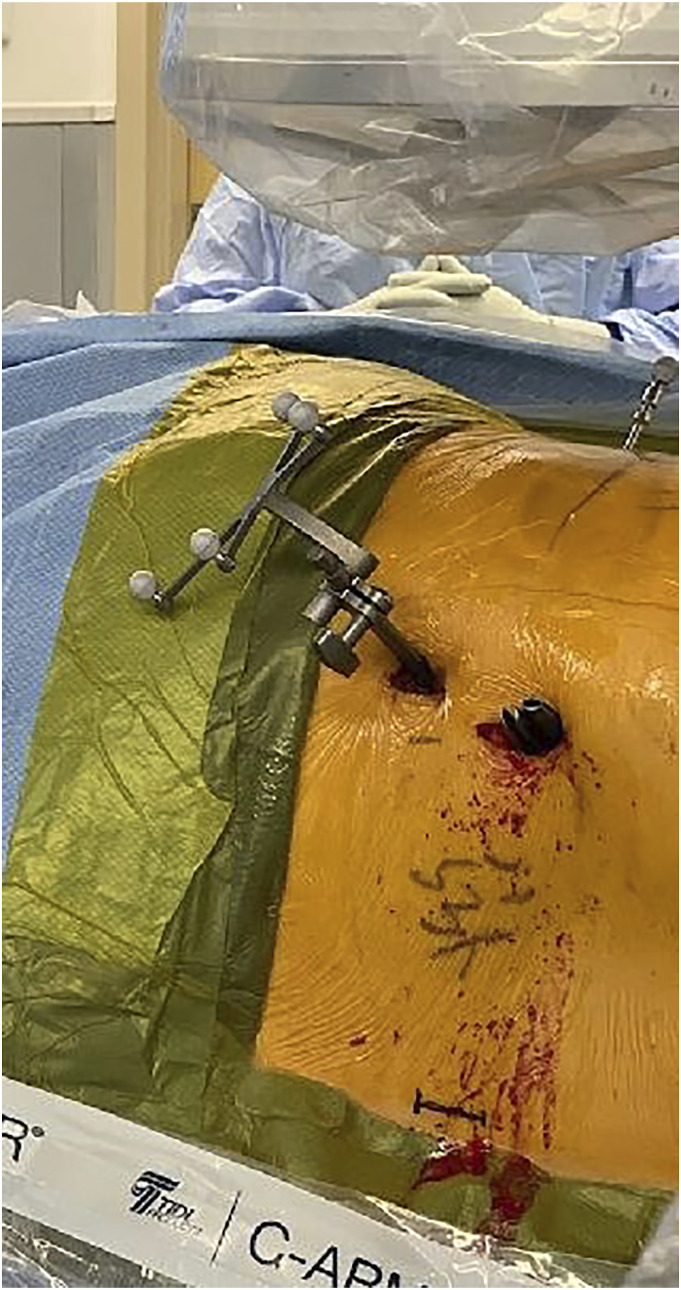
Figure 1.
Ipsilateral screws have been placed. The incision for the contralateral screws is marked just above the inferior border of the prepped area. When the incision is so close to the edge of the drape, care must be taken to avoid contamination with un-prepped skin. This patient had a significant amount of posterior soft tissue that made the skin incisions very lateral due to triangulation. The DRB guide pin is inserted into left PSIS and the surveillance marker is in the lateral iliac crest.
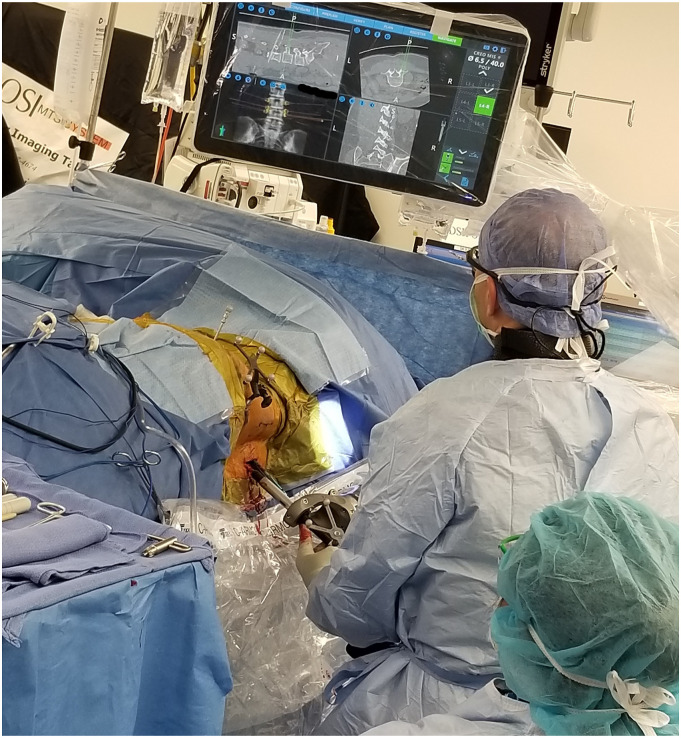
Figure 2.
Drilling the opening in the pedicle for screw placement with a navigated drill. The monitor shows real-time position of the drill in the spine. The horizontal bar on the bottom right corner of the monitor shows the amount of lateral force being applied to the end-effector to monitor for skiving of the drill off the planned trajectory.
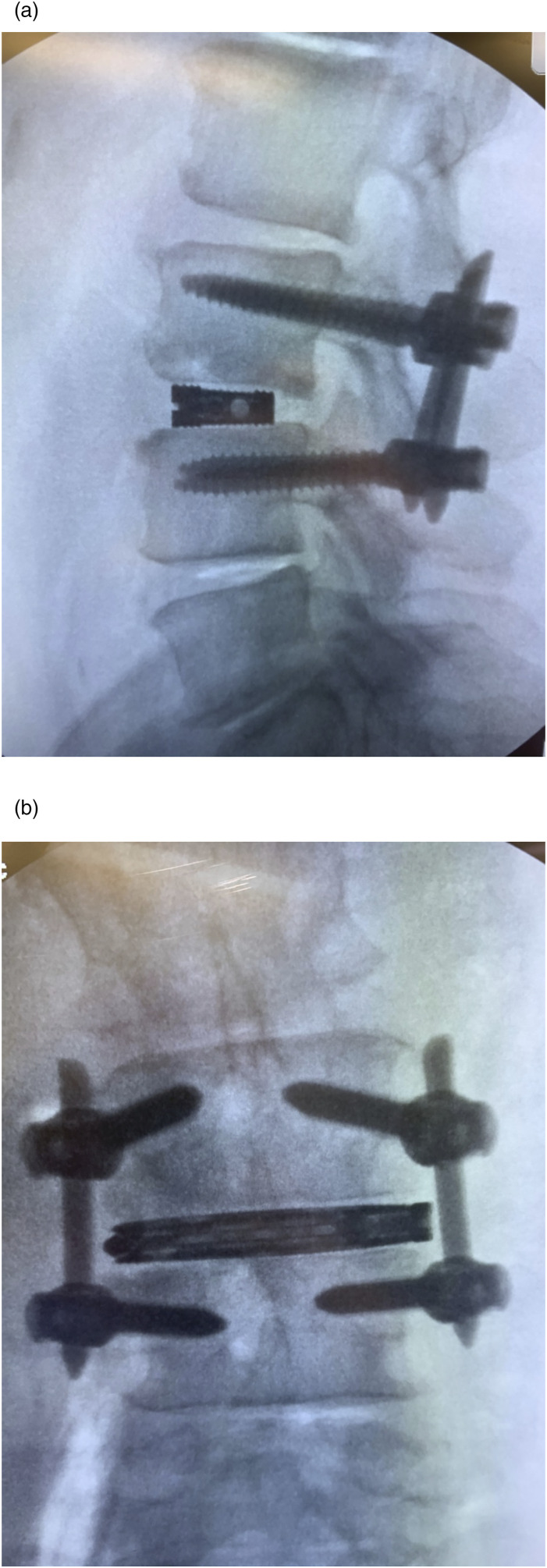
Figure 4.
Intraoperative fluoroscopy images show posterior hardware and interbody spacer placement after robotic-assisted single position LLIF with posterior fusion and instrumentation.
Limitations
Limitations of this study include small sample size, lack of control arm with patients having traditional open pedicle screw placement in prone position, lack of post-operative CT scans to assess screw placement more accurately, and lack of recorded time it took to set up the navigation and place the screws intraoperatively. While the follow-up time was only 3 months, it was considered adequate for the study evaluating the novel method of pedicle screw placement. It is very unlikely for a misplaced pedicle screw to manifest clinically beyond 3 months after lumbar fusion surgery.
Summary
Single-position LLIF procedure using robotic assistance allows for efficient and accurate pedicle screw insertion without the need to reposition the patient prone in the middle of the case. In this small retrospective cohort review, there were no observed complications intraoperatively and no hardware mal-positions that required revision surgery. Robotic-assisted single-position LLIF procedure with posterior instrumentation is safe, effective, and reproducible when proper technique steps are followed.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Vladimir Sinkov https://orcid.org/0000-0003-1429-7119
Bryan W. Cunningham https://orcid.org/0000-0002-4604-395X
References
- 1.Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: A report from the scoliosis research society morbidity and mortality committee. Spine. 2011. Apr 1;36(7):556-563. [DOI] [PubMed] [Google Scholar]
- 2.Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without workers' compensation claims treated with minimally invasive or open transforaminal lumbar interbody fusion. Spine. 2012. Oct 15;37(22):1914-1919. [DOI] [PubMed] [Google Scholar]
- 3.Ge DH, Stekas ND, Varlotta CG, et al. Comparative Analysis of Two Transforaminal Lumbar Interbody Fusion Techniques: Open TLIF Versus Wiltse MIS TLIF. Spine. 2018. Oct 15;44(9):E555-E560. [DOI] [PubMed] [Google Scholar]
- 4.Ryu DS, Ahn SS, Kim KH, et al. Does minimally invasive fusion technique influence surgical outcomes in isthmic spondylolisthesis? Minim Invasive Ther Allied Technol. 2018. Sep;28(1):33-40. [DOI] [PubMed] [Google Scholar]
- 5.Phan K, Hogan JA, Mobbs RJ. Cost-utility of minimally invasive versus open transforaminal lumbar interbody fusion: Systematic review and economic evaluation. Eur Spine J. 2015. Nov;24(11):2503-2513. [DOI] [PubMed] [Google Scholar]
- 6.Singh K, Nandyala SV, Marquez-Lara A, et al. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J. 2014. Aug 1;14(8):1694-1701. [DOI] [PubMed] [Google Scholar]
- 7.Mummaneni PV, Bisson EF, Kerezoudis P, et al. Minimally invasive versus open fusion for grade I degenerative lumbar spondylolisthesis: analysis of the quality outcomes database. Neurosurg Focus. 2017. Aug;43(2):E11. [DOI] [PubMed] [Google Scholar]
- 8.Tay KS, Bassi A, Yeo W, Yue WM. Intraoperative reduction does not result in better outcomes in low-grade lumbar spondylolisthesis with neurogenic symptoms after minimally invasive transforaminal lumbar interbody fusion-a 5-year follow-up study. Spine J. 2016. Feb;16(2):182-190. [DOI] [PubMed] [Google Scholar]
- 9.Tay KS, Bassi A, Yeo W, Yue WM. Associated lumbar scoliosis does not affect outcomes in patients undergoing focal minimally invasive surgery-transforaminal lumbar interbody fusion (MISTLIF) for neurogenic symptoms-a minimum 2-year follow-up study. Spine J. 2017. Jan;17(1):34-43. [DOI] [PubMed] [Google Scholar]
- 10.Wu MH, Dubey NK, Li YY, et al. Comparison of minimally invasive spine surgery using intraoperative computed tomography integrated navigation, fluoroscopy, and conventional open surgery for lumbar spondylolisthesis: A prospective registry-based cohort study. Spine J. 2017. Aug;17(8):1082-1090. [DOI] [PubMed] [Google Scholar]
- 11.Mueller K, Zhao D, Johnson O, Sandhu FA, Voyadzis JM. The difference in surgical site infection rates between open and minimally invasive Spine surgery for degenerative lumbar pathology: A retrospective single center experience of 1442 cases. Oper Neurosurg (Hagerstown). 2018. Aug 9;16(6):750-755. [DOI] [PubMed] [Google Scholar]
- 12.Price JP, Dawson JM, Schwender JD, Schellhas KP. Clinical and radiologic comparison of minimally invasive surgery with traditional open transforaminal lumbar interbody fusion. Clin Spine Surg. 2018. Mar;31(2):E121-E126. [DOI] [PubMed] [Google Scholar]
- 13.Kim CW. Scientific basis of minimally invasive Spine surgery. SPINE. 2010;35:S281-S286. Number 26S. [DOI] [PubMed] [Google Scholar]
- 14.Buckland AJ, Ashayeri K, Leon C, et al. Single position circumferential fusion improves operative efficiency, reduces complications and length of stay compared with traditional circumferential fusion. The Spine Journal. 2020;21(5):810-820. [DOI] [PubMed] [Google Scholar]
- 15.D'Souza M, Gendreau J, Feng A, Kim LH, Ho AL, Veeravagu A. Robotic-assisted Spine surgery: History, efficacy, cost, and future trends. Robot Surg. 2019. Nov 7;6:9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443. [DOI] [PubMed] [Google Scholar]
- 17.Campbell PG, Nunley PD, Cavanaugh D, Kerr E, Utter PA, Frank K, Stone M. Short-term outcomes of lateral lumbar interbody fusion without decompression for the treatment of symptomatic degenerative spondylolisthesis at L4-5. Neurosurg Focus. 2018. Jan;44(1):E6. [DOI] [PubMed] [Google Scholar]
- 18.Guiroy A, Carazzo C, Camino-Willhuber G, et al. Single-position surgery versus lateral-then-prone-position circumferential lumbar interbody fusion: A systematic literature review. World Neurosurg. 2021;151:e379-e386. [DOI] [PubMed] [Google Scholar]
- 19.Ouchida J, Kanemura T, Satake K, Nakashima H, Ishikawa Y, Imagama S. Simultaneous single-position lateral interbody fusion and percutaneous pedicle screw fixation using O-arm-based navigation reduces the occupancy time of the operating room. European Spine Journal. 2020;29(6):1277-1286. doi: 10.1007/s00586-020-06388-6. [DOI] [PubMed] [Google Scholar]
- 20.Hiyama A, Katoh H, Sakai D, Sato M, Tanaka M, Watanabe M. Comparison of radiological changes after single- position versus dual- position for lateral interbody fusion and pedicle screw fixation. BMC Musculoskelet Disord. 2019;20(1):601. doi: 10.21203/rs.2.16118/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziino C, Konopka JA, Ajiboye RM, Ledesma JB, Koltsov JCB, Cheng I. Single position versus lateral-then-prone positioning for lateral interbody fusion and pedicle screw fixation. J Spine Surg. 2018;4(4):717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen K, Thastum M, Nørholt SE, Blomlöf J. Relative blood loss and operative time can predict length of stay following orthognathic surgery. Int J Oral Maxillofac Surg. 2016;45(10):1209-1212. [DOI] [PubMed] [Google Scholar]
- 23.Mahadevan D, Challand C, Keenan J. Revision total hip replacement: Predictors of blood loss, transfusion requirements, and length of hospitalisation. J Orthop Traumatol. 2010;11(3):159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blizzard DJ, Thomas JA. MIS single-position lateral and oblique lateral lumbar interbody fusion and bilateral pedicle screw fixation. Spine. 2018;43(6):440-446. [DOI] [PubMed] [Google Scholar]
- 25.Huntsman KT, Riggleman JR, Ahrendtsen LA, Ledonio CG. Navigated robot-guided pedicle screws placed successfully in single-position lateral lumbar interbody fusion. J Robot Surg. 2020;14(4):643-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahern DP, Gibbons D, Schroeder GD, Vaccaro AR, Butler JS. Image-guidance, robotics, and the future of Spine surgery. Clinical spine surgery. 2020;33(5):179-184. [DOI] [PubMed] [Google Scholar]
- 27.Louw DF, Fielding T, McBeth PB, Gregoris D, Newhook P, Sutherland GR. Surgical robotics: A review and neurosurgical prototype development. Neurosurgery. 2004;54(3):525-537. doi: 10.1227/01.neu.0000108638.05274.e9. [DOI] [PubMed] [Google Scholar]
- 28.Vaccaro AR, Hussain M, Harris J, et al. In vitro analysis of accuracy, dosage and surgical time required for pedicle screw placement using conventional percutaneous screw and robotic-assisted screw techniques. The Spine Journal. 2017;17(10):S261. doi: 10.1016/j.spinee.2017.08.191. [DOI] [Google Scholar]