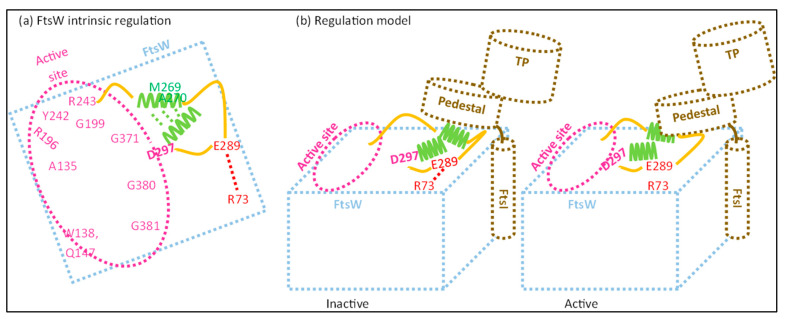
Figure 6.
Intrinsic FtsWI regulation. FtsWI appear to have an intrinsic regulatory mechanism, which is expected to be affected by regulators. In a simplified scheme of the periplasmic side of FtsW (a), the catalytic residue D297 is located at the periphery of the active side (purple–dotted ring) in the loop between TM7 and TM8 that also contains the inhibiting E289 residue and the activating M269 and A270 residues. A balance between these two regions on the same loop appear to regulate FtsW GTase activity. A putative interaction between E289 and R73, located on TM1, likely destabilizes or sequesters D297 from the rest of the active site, leading to an inactive conformation. The hydrophobic region around M269 and A270 may antagonize this and lead to the stable positioning of D297 in the active site. (b) A speculative model describing the possible activation of FtsW by FtsI is shown. Regions of the pedestal domain of FtsI may interact with the α-helix containing M269 and A270, pushing the regulatory loop in an active conformation.