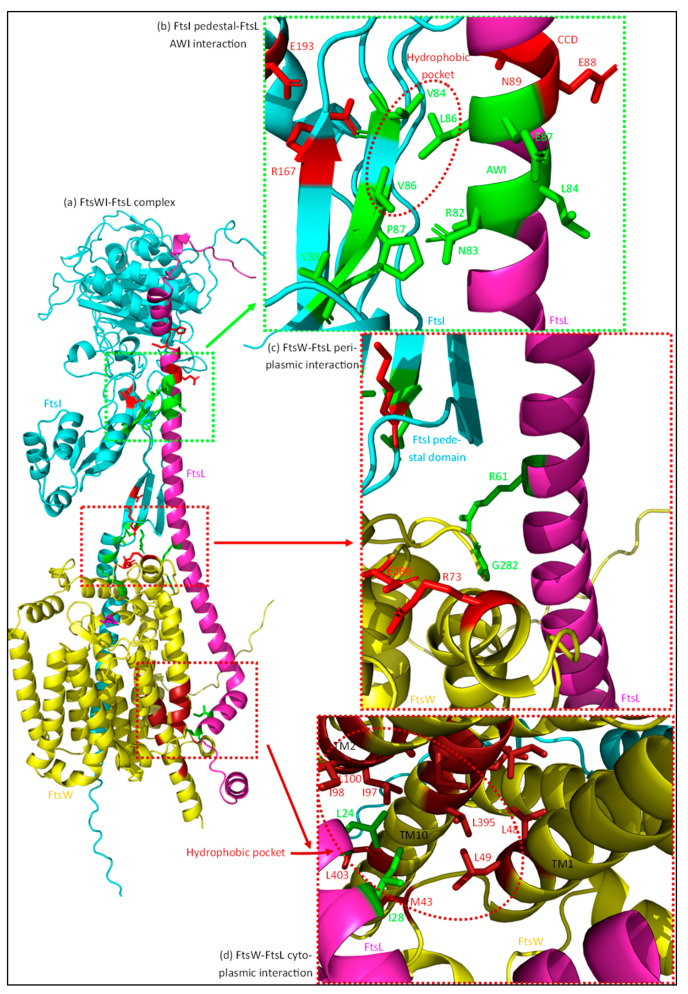
Figure 12.
Interactions between FtsL and FtsWI. FtsL interacts with multiple regions of FtsWI, which likely leads to a stable active conformation. (a) The complex formed between FtsW (yellow), FtsI (cyan) and FtsL (purple) is shown. (b) A periplasmic interaction between the FtsL AWI domain (green) and the pedestal domain of FtsI is predicted to be important for FtsWI activation. A hydrophobic pocket around FtsI residues V84 and V86 (red dotted ellipse) appears to interact with FtsL residue L86. (c) FtsL also appears to interact with FtsW close to the IM on the periplasmic side. A hydrogen bond between FtsL residue R61 and FtsW residue G282 is formed in the predicted structure. The R61–G282 interaction (green) may stabilize the periplasmic loop in a more active conformation. (d) FtsL recruits FtsW through its cytoplasmic domain and this interaction also plays a minor role in FtsW activation. A hydrophobic pocket formed by residues om TM2 and TM10 of FtsW (brick-red) interacts with the FtsL cytoplasmic domain (L24, I28; green), possibly stabilizing the TM domains that carry active site-residues at the periplasmic side. The structures shown here are predictions produced with AlphaFold2 advanced, and visualized with PyMOL Molecular Graphics System, Version 2.5.2 Schrödinger, LLC. Sequences for the prediction were obtained from the UniProtKB database (P0AEN4-1 for FtsL, P0ABG4-1 for FtsW, P0AD68-1 for FtsI).