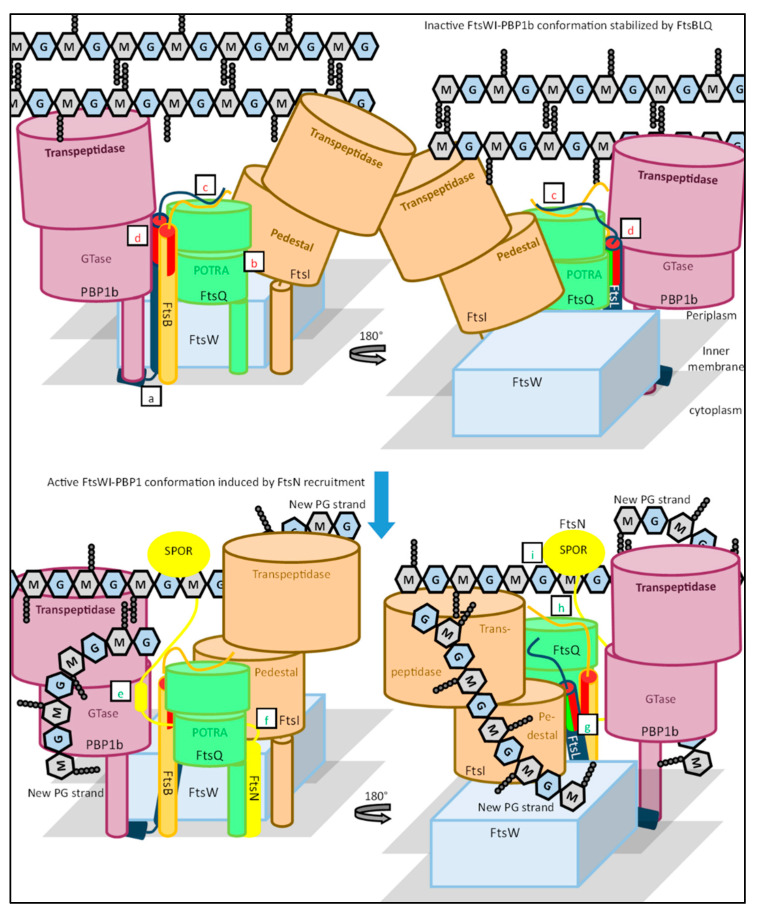
Figure 16.
Regulation of FtsWI–PBP1b by FtsBLQ and FtsN. A model is proposed where FtsBLQ and FtsN stabilize the FtsWI–PBP1b septal peptidoglycan synthesis machinery in active or inactive conformations based on certain allosteric interactions. These interactions are numbered and either suppress (red), increase (green), or are neutral (black) regarding sPG synthesis. (a) FtsW/L cytoplasmic interaction. This interaction between FtsL and FtsW in the cytoplasm recruits the synthesis machinery to the division site. While primarily involved in recruitment, the interaction may also be involved in the stabilization of FtsW in a more active conformation. (b) FtsQ–FtsWI. The membrane proximal part of the FtsQ POTRA domain seems to interact with a periplasmic loop of FtsW (between TM1 and TM2) and the pedestal domain of FtsI. (c) C-terminal FtsBLQ interaction. A strong periplasmic interaction between FtsB and FtsQ is important for the stability of the FtsBL coiled coil structure, as well as an additional C-terminal FtsB–FtsL interaction that occurs in the same region. This increased stability is thought to stabilize the whole complex in an inactive conformation, thus negatively affecting sPG synthesis. (d) PBP1b–FtsL. A direct interaction between PBP1b and the CCD domain of FtsL (red) suppresses PBP1b GTase activity. (e) PBP1b–FtsN. FtsN (yellow) also directly interacts with the same PBP1b region, outcompetes FtsL–CCD, and leads to the activation of PBP1b GTase activity. (f) FtsN–FtsQ. FtsN is proposed to interact with the same FtsQ region as FtsI and leads to dissociation of the inhibitory FtsQ–FtsWI interaction. (g) FtsL–FtsI. An interaction between the now available FtsL–AWI domain (green) and the pedestal domain of FtsI stabilizes FtsWI in a highly active conformation, starting septal peptidoglycan synthesis. (h) C-terminal FtsBLQ (in)stability. An interaction between FtsN and the C-terminal domain of FtsQ (around residue S242) is proposed to lead to instability in the C-terminal FtsBLQ interaction through a conformational change in FtsQ. Together with interactions (e,f), the instability in the FtsBL coiled coil domain is increased, which leads to dissociation of the upper part of the coiled coil and subsequently to the activating FtsL–FtsI interaction (see interaction (g)). (i) FtsN SPOR interacting with denuded glycans. The SPOR domain of FtsN binds to denuded glycan as shown, and most likely directs the synthesis complex to ‘open’ PG, where synthesis can occur. New PG strands are formed when the whole synthesis complex is in its active state, induced by the sum of activating interactions (green). This model is not on scale and does not represent the possible stoichiometry of a complete synthesis node. M and G represent NAM and NAG residues, respectively.