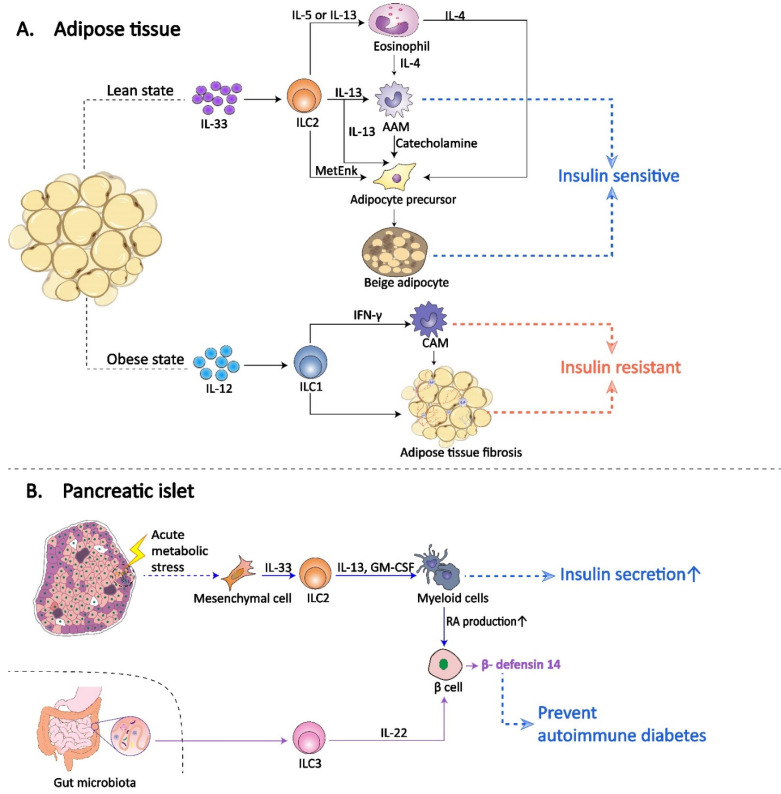
Figure 2.
Role of ILCs in diabetes mellitus. A | ILCs in adipose tissue. In the lean state, IL-33 induces adipose-resident ILC2s to produce the cytokines IL-5 or IL-13, which support the recruitment and accumulation of eosinophils in AT. Eosinophils produce IL-4 to sustain and recruit AAMs. ILC2s produce ample IL 13 and may also directly contribute to AAM recruitment and maintenance. AAM byproducts, such as IL-10, contribute to adipocyte insulin sensitivity and protect against DM. In addition, IL-4, IL-13 and methionine-enkephalin peptides (MetEnk) and catecholamines, produced by eosinophils, ILC2s and AAMs, respectively, promote the proliferation and differentiation of adipocyte precursors into beige adipocytes. Beige fat biogenesis also promotes insulin sensitivity and prevents DM. In the obese state, while IL-12 promotes the selective accumulation of adipose-resident ILC1s. ILC1s drive CAM polarization by IFN-γ production and promote AT fibrosis, contributing to obesity-associated insulin resistance and DM. B | ILCs in pancreatic islets. In diabetic or obese states, the islets are also in an inflammatory background. IL-33 is produced by mesenchymal cells as a stress signal in islets. As the main IL-33-responsive cells in islets, islet-resident ILC2s stimulate the capacity of myeloid cells to produce RA, which in turn enhances insulin secretion in islet β cells and protects against DM. In the gut, the microbiota controls IL-22 expression by ILC3s within pancreatic islets through different pathways. ILC3-derived IL-22 induces islet β cells to produce β-defensin, preventing autoimmune diabetes.