Fig. 1.
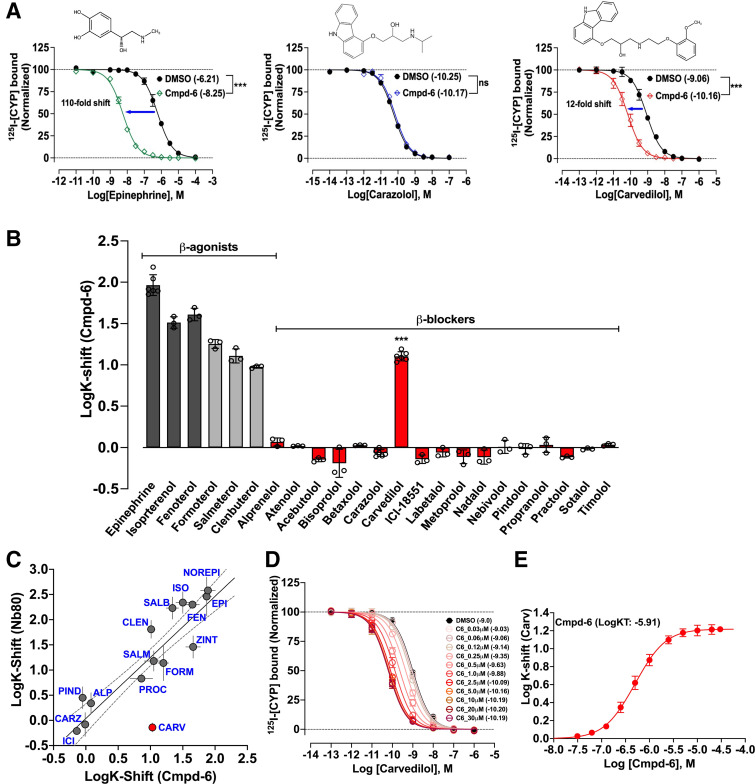
Cmpd-6 is positively cooperative with the β-blocker carvedilol. (A) Radioligand competition binding showing the displacement of 125I-CYP by the cold competitor’s epinephrine, carazolol, and carvedilol respectively at the β2AR in HDL. Data showing left-shifts in epinephrine (green) and carvedilol (red) competition curves indicate positive cooperativity with cmpd-6. Chemical structures of competing ligands are shown on top of respective data panel. Points on the curves represent normalized cpm values from three independent experiments ± S.D. (B) Bar graph showing cmpd-6–mediated affinity shifts (K-shifts), reported as the difference in LogIC50 (cmpd-6 versus DMSO) for a diverse panel of β-ligands: full agonists (gray), partial agonists (light gray), and anatgonists (red). Data represent shifts in LogIC50 (LogK-shifts) values derived from three to six independent experiments ± S.D. (C) Correlation plot showing comparison of affinity-shifts (LogK-shifts) for a panel of β-ligands mediated by Nb80 (Staus et al., 2016) and cmpd-6 (this study). Dashed lines around the line of correlation (solid gray) represent the 95% confidence interval. Carvedilol (red) is the one outlier ligand that is uniquely cooperative with only cmpd-6 but not Nb80. (D) Cmpd-6 dose response curves obtained by displacement of 125I-CYP by the cold carvedilol. (E) Curve showing shifts in LogIC50 (ΔLogIC50) of carvedilol mediated by cmpd-6 dose response; derived from data in (D). Points on the curves represent normalized cpm (D) and ΔLogIC50 (E) values from four independent experiments ± S.D. Statistical comparisons were done using one-way ANOVA with Bonferroni’s post hoc test. ***P < 0.001; ns, not significant.