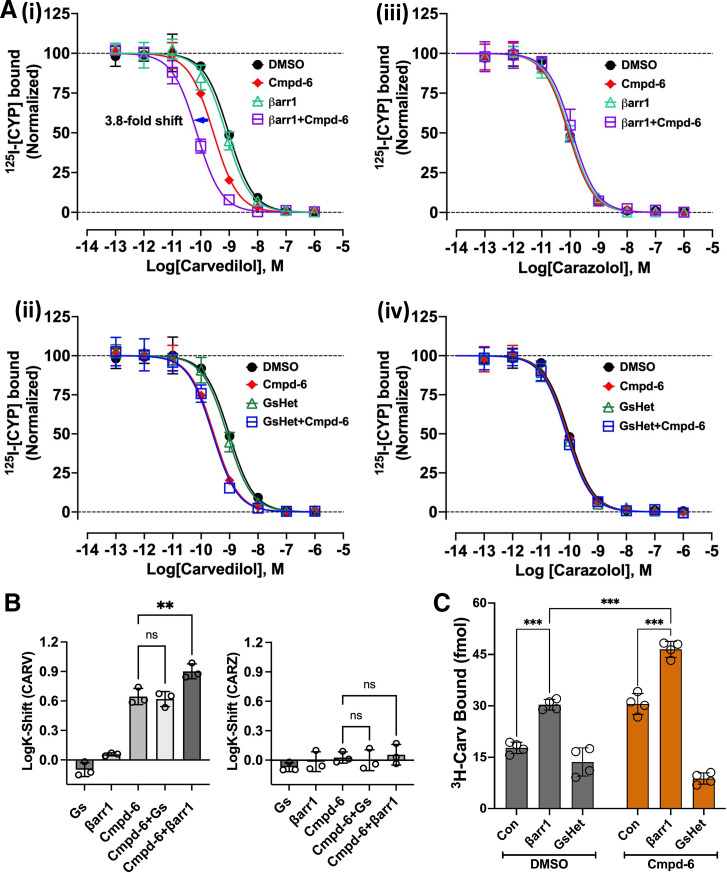
Fig. 2.
Cmpd-6 and β-arrestin1–mediated high-affinity binding of carvedilol. (A) Radioligand competition binding showing the displacement of 125I-CYP ([125I]-Cyanopindolol) by the cold competitors carvedilol [(i) and (ii)] and carazolol [(iii) and (iv)], respectively, at the β2V2RpP in HDL. Cmpd-6 is shown to display cooperative effects with βarr1 (i), but not with Gs (ii), in promoting high-affinity left-shifts of carvedilol competition curves. No such cooperativity is observed in carazolol competition curves. (B) Bar graph showing comparison of CARV (carvedilol) and CARZ (carazolol) affinity-shifts (LogK-shifts) mediated by cmpd-6. Data shown in (A) and (B) represent values obtained from three independent experiments ± S.D. (C) Bar graph showing the direct high-affinity binding of 3H-Carv to β2ARpP in HDL. Compared with DMSO control (Con), cmpd-6 alone and together with βarr1, but not Gs, is shown to potentiate 3H-Carv binding. Data shown in the bar graphs represent mean receptor binding values obtained from four independent experiments ± S.D. Statistical comparisons were made by two-way ANOVA followed by Bonferroni’a post hoc test. **P < 0.01; ***P < 0.001; ns, not significant.