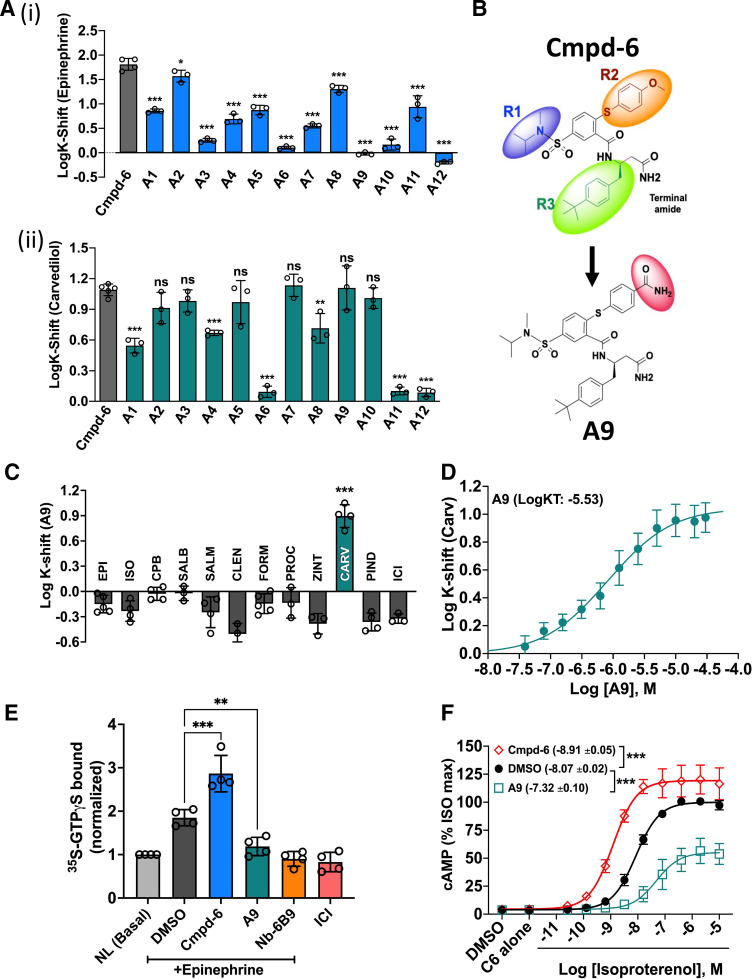
Fig. 5.
Cmpd-6 analog A9 displays carvedilol-specific cooperativity at the β2AR. (A) Bar graph showing affinity shifts (LogK-shifts) of epinephrine (i) and carvedilol (ii) mediated by cmpd-6 (gray bars) or its analogs (A1–A12, colored bars) compared with DMSO control. (B) Chemical structures of cmpd-6 and its analog A9 highlighting the modified R2 moiety in the analog. (C) Bar graph showing the allosteric cooperative effects of A9 on affinity shifts of a panel of β-ligands (agonists and antagonists). A9 shows positive cooperativity only with carvedilol compared with other ligands tested. Bar graphs in (A) and (C) show mean LogK-shifts ± S.D. obtained from three to five independent experiments. (D) Curve showing affinity shifts in LogIC50 (LogK-Shift) for carvedilol mediated by a dose of A9. Points on the curves represent LogK-Shift values derived from three independent competition binding experiments ± S.D. (E) Bar graph showing binding of 35S-GTPγS to heterotrimeric Gs either under basal, no ligand (NL) condition or after epinephrine stimulation of the β2AR in HDL. Data for respective conditions (n = 4) are normalized to basal (unstimulated) binding of 35S-GTPγS. (F) Curves showing the effect of cmpd-6 and A9 on isoproterenol (ISO)–stimulated cAMP generation. Cells were pretreated for 15–20 minutes with either cmpd-6 or A9 (30 µM) and then stimulated with a dose of ISO. The amount of cAMP production by endogenously expressed β2AR was measured 10 minutes after ISO stimulation. Curve fits were generated in GraphPad Prism with data points obtained from five independent experiments done in duplicate. Each data point was normalized to the maximal level of ISO-induced activity in the vehicle (DMSO) control, expressed as a percentage, and represents mean ± S.D. Statistical comparisons were made using one-way ANOVA with Bonferroni’s post hoc tests. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.