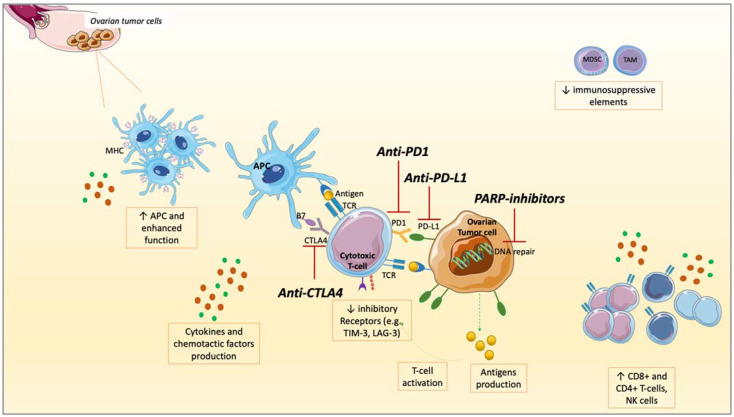
Figure 2.
The interplay between PARP inhibitors (PARPis) and immune checkpoint inhibitors (ICIs): modifications of soluble factors and cell of tumor microenvironment (TME) in ovarian cancer (OC). After the administration of PARPis, a series of modifications occur: an increased production and surface exposure of antigens on tumor cells, increased PD-L1 expression, production of cytokines, and chemotactic factors. Antigen-presenting cells (APCs) are enhanced in their function with major histocompatibility complex (MCH) up-regulation and increased in numbers. A higher number of CD4+ and CD8+ T-cells, and NK cells, are recruited at tumor sites, resulting in higher TILs and immune response activation. On the other hand, immune-suppressive elements such as Myeloid-derived suppressor cells (MDCSs) and Tumor-associated macrophages (TAMs) are reduced, as well as inhibitory receptors (such as T-cell membrane protein 3 [TIM-3], Lymphocyte-activation gene 3 [LAG-3]). These modifications shift the TME toward a higher immune responsivity. Targeting Programmed Death-Ligand 1 (PD-L1), PD1, or Cytotoxic T-lymphocytes Associated Protein 4 (CTLA4), ICIs unleash the anti-tumor immune response, potentiating the immune activation against tumor cells. Therefore, combining these two drug classes could result in a higher anti-tumor immune response in OC.