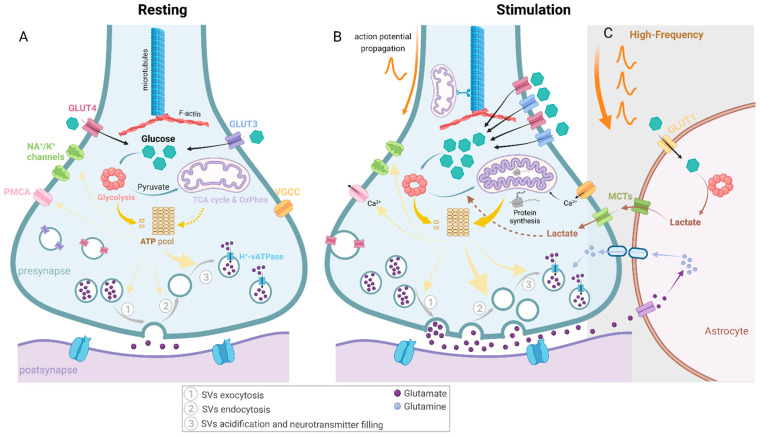
Figure 2.
The dynamic energy requirements and fulfilments of presynapses upon distinct activation states. Glucose is the main energy substrate in the brain and given that synapses are the most energy-demanding sites in the brain, glucose degradation is important to provide ATP for synaptic pathways. Glucose is imported through glucose transporters (GLUT3 and GLUT4) and can be degraded through glycolysis in the cytosol and generate pyruvate and, ultimately, lactate. Pyruvate can be imported to mitochondria where, through multiple reactions shown in Figure 1, it will generate higher yields of ATP but at a lower rate than glycolysis [6]. Multiple reactions at presynapses require ATP, namely, synaptic vesicle recycling, endocytosis, acidification and neurotransmitter filling, priming (not represented in this figure) and exocytosis. Additionally, several ion channels in the presynaptic membrane require ATP, including sodium-potassium voltage-gated channels (Na+/K+ channels) and plasma membrane Ca2+-ATPase (PMCA), necessary to extrude Ca2+ that accumulates inside presynapses. (A) In a resting state, minimal energy is necessary to fuel these membrane ion channels [39], and it is speculated that due to the lack of stimuli, the energetic burden of synaptic vesicles’ (SVs) endo- and exocytosis is also reduced, despite some spontaneous release. Recently, in rat hippocampal neurons, it was suggested that SV re-acidification (through H+-vATPases) was the major energy consumer in presynapses (~44%) [39]. (B) Upon synaptic stimulation, the demand for energy is increased. Although the energy proportion required for each synaptic ATP-dependent process is not yet defined, SV endocytosis is assumed to be one of the most energy-demanding processes. Both glycolysis and OxPhos have been shown to be important to meet the activity-driven energy requirements. In active synapses, mitochondria present a higher volume and an increased cristae density [44]. Additionally, translation of both mitochondrial [45]- and nuclear-encoded mitochondrial [46] proteins have been shown to be increased upon synaptic activity. Additionally, recruitment of axonal mitochondria has also been shown to be important upon synaptic energy stress [47]. (C) Despite the controversy of the astrocyte-neuron lactate shuttle (ANLS) hypothesis, astrocytic lactate has been important to maintain synaptic transmission under high-frequency stimuli (HFS) [23,48,49]. Under these conditions, augmented glutamate levels in the glutamatergic synaptic clefts might boost astrocytic glutamate uptake to reconvert it back to glutamine. Concomitantly, astrocytic glycolysis is increased, generating lactate, which will be delivered to neurons through monocarboxylate transporters (MCTs) present in both neuronal and astrocytic membranes. This lactate will complement neuronal glycolysis and OxPhos in fulfilling the high SV endocytosis resultant from HFS.