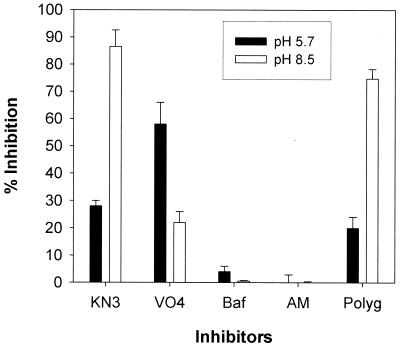
FIG. 6.
Effect of a panel of standard inhibitors and polygodial against membrane-bound ATPases in S. cerevisiae at pH 5.7 (filled bars) and pH 8.5 (open bars). Whole membranes were used, and the amount of enzyme activity ranged from 0.7 to 1.1 U (nanomoles of Pi per minute), with specific activity ranging from 50 to 90 U/mg. The inhibition ratio (percentage) was calculated as follows: {1 − [(I − C2)/(C1 − C2)] × 100}, where C1 is enzyme alone, I is enzyme plus inhibitor, and C2 is inhibitor without enzyme (10 mM ATP present in all). Inhibitors were used at concentrations known to give maximal inhibition of their selective enzyme. KN3, potassium azide at 5 mM; VO4, sodium orthovanadate at 100 μM; Baf, bafilomycin at 50 nM; AM, ammonium molybdate at 0.2 mM; Polyg, polygodial at 50 μg/ml. Values are means ± standard deviations.